1 Introduction
Indian mulberry or Noni (Morinda citrifolia L.; Rubiaceae) is a native of Andaman and Nicobar Islands (India), Indonesia and adjoining Indo-Pacific region (Singh et al., 2010; McClatchey, 2002). The genus Morinda has more than 160 species mostly distributed all across the tropical island regions (Razafimandimbison et al., 2009). The M. citrifolia has been identified as an important species with wide range of health and commercial uses (Singh et al., 2010; Singh et al., 2011a). Its health benefits can be viewed from the fact that since 1976, about 19 patents have been registered by the US Patent and Trademark Office in United States of America (USPTO, 2005) and Noni juice has been accepted in the European Union as a novel food (European Commission Scientific Committee of Food, 2005). Even though, price of Noni based products in Indian markets is still high but they got significant reach in rich and upper middle class of public due to health benefiting perceptions. Around 200 phyotochemicals were reported in M. citrifolia which includes phenolics, carotenoids, polysaccharides, flavonoids, iridoids, fatty acids, scopoletin, catechin, β-sitosterol, damnacanthal and alkaloids which contribute in good health of the body (Singh et al., 2010). In fact mixture of compounds in plant extract are more effective than single isolated compound for health benefits (Rasoanaivo et al., 2011) hence, knowledge of plant species or genotypes rich in major groups of compounds could be of more use in herbal industry.
The Andaman and Nicobar Islands (6°N to 14°N and 92°E to 94°E) in Bay of Bengal have rich biodiversity of tropical flora and fauna and also have 223 endemic plant species (Balakrishnan and Ellis, 1996). The M. citrifolia is commonly used in traditional medicines by indigenous tribes of the islands for curing ailments such as fever, stomach ache, high blood pressure, wound and injuries, joint pain, headache, bone fracture, headache, worms and mosquito repellent (McClatchey, 2002). Investigations showed significant levels of species diversity in genus Morinda viz a viz great extent of genetic diversity in M. citrifolia (Singh et al., 2011b; Singh et al., 2012), a species of most commercial significance. However, little is known about variation in phyto-constituents in available diversity for use for breeding and in herbal industry for extraction purpose. It is well accepted that the concentration of phytochemicals is greatly influenced by genotype factor while growing environment, plant tissues and estimation method also contribute in variation (Rethinam and Sivaraman, 2007; Singh et al., 2014).
Noni fruits are bitter and extremely off-flavoured and strictly undergoes for processing steps to bring the acceptable products. For this, the knowledge of genotypes with superior fruit traits for processing purpose is essential for sustainable growth of the prospective Noni based industry. Genotypes with higher recovery of pulp and juice are essential for cosmetic and herbal industry while excess share of seed is useful for extraction of oils rich in fatty acids. Hence, identification of Noni genotypes having superior fruit traits and rich in health benefiting phyto-constituents was pre-requisite in preparation of Noni based herbal products. Therefore, the present study was conducted to analyze phyto-nutrients and observe fruit traits in 33 promising genotypes of M. citrfofila collected from all across the Andaman and Nicobar Islands.
2 Results
2.1 Observation of fruit traits
The observation on fruit traits revealed significant variation in the tested 33 genotypes of M. citrifolia (Figure 1). Average fruit weight was ranged from 50.5 to 117.9 g, lowst in CHTAP-13 and highest in JGH-5. Number of seeds per plant (90.0-221.0) and pulp recovery (35.0-50.0%) also had significant variation in the genotypes. The superior genotypes for pulp recovery were TRA-2, WAND-4, TRA-1 and CAL-10 and for higher recovery of fresh seeds were SPG-2, MEM-3, MEM-2 and HD 6A. Although, some of the genotypes bears some very big size fruits (upto 350 g) as also reported by Rethinam and Sivaraman (2007) but average fruit weight was highest in JGH-5 (117.9 g) followed by GAH-2 (112.4 g), HD-6 (110.9 g) and MHP-19 (108.4 g). TRA-1 and TRA-2 had small fruits but performed well in overall observations.
|
.png)
Figure 1 Fruits of Noni (Morinda citrifolia L.) - at harvest (a) and processing stage (b)
|
2.2 Phytochemicals
Phytochemicals are important source of bioactive compounds from plant kingdom and play key role in human health and nutrition. Now-a-days, it has been accepted that these compounds are key factors for longevity of plants and human as they neutralize, scavenge or inhibit the free radicals generated as by-products of biochemical reactions in body system. The M. citrifolia genotypes were observed as rich source of such natural antioxidants.
|
.png)
Table 1 Details of 33 genotypes of Morinda citrifolia L. and their phytochemical contents (mg/100 g fresh weight)
|
The results of present study for phyto-constituent analysis in 33 genotypes of M. citrifolia are presented in Table 1. Overall, the carotenoids (455.2 mg/100 g) follower by flavonoids (344.1 mg/100 g) and polyphenol (261.8 mg/100 g) were predominant phytochemicals in M. citrifolia fruits. The polyphenol analysis showed significant (p<0.05) variation among in tested genotypes which ranged from 165.40 (TRA-1) to 370.26 mg/100 g (MBAY-6). Other polyphenol rich genotypes were identified as CHTAP-13 (365.9 mg/100 g), HD-6 (365.2 mg/100 g) and HD-6A (347.0 mg). M. citrifolia genotypes showed significant variations for flavonoid content which ranged from 47.57 to 656.18 mg/100 g, maximum in JGH-5 while minimum in CHTAP-13. The potential genotypes for flavonoid were identified as JGH-5 (656.2 mg/100 g), TRA-1 (614.0 mg/100 g), PBAY-7 (599.4 mg/100 g) and TRA-2 (588.2 mg/100 g). Greater variation was observed in M. citrifolia genotypes for tannin content which ranged from 88.92 mg /100 g (MEM-3) to 395.20 mg/100 g (HD-6). TRA-1, CHTAP-13, HD-6A, JGH-1 and JGH-5 were also rich source of tannin content. The study observed significant (p<0.05) difference among the tested genotypes for anthocyanin content which was highest in MANJ-1 (340.39 mg/100 g), and lowest in MEM-3 (163.25 mg/100 g). The carotenoids content in M. citrofolia genotypes ranged from 114.68 to 696.26 mg/100 ml, the highest in LH-12 while lowest in NESAH-15. The genotypes LH-12 (696.3), AHD-1 (678.8 mg/100 g), TRA-2 (663/4 mg/100 g) were also found to be rich in carotenoids. However, M. citrifolia genotypes did not show significant variation for ascorbic acid content which was ranged from 71.0 mg/100 g (MBAY-16) to 98.20 mg/100 g (GAH-1). The findings are in the line of earlier reports on M. citrifolia (Singh et al., 2011a; USPTO, 2005; Sandeep et al., 2015). However, variations might be due to difference in genotypes, environment and estimation methods used in present and referred studies. It got support from the fact that the present study had similarity in environmental factors in Germplasm Block, sampling of mature fruits and estimation method for phyto-constituents were same except genotypes. The findings support the previously revealed DNA marker based genetic diversity in M. citrofila (Singh et al., 2011b; Singh et al., 2012) which have promise in tapping diversity for breeding of genotypes rich in phyto-nutrients.
The phytochemicals are secondary metabolites which play important role in plant defence, photosynthesis and reproductive mechanisms. Their synthesis is affected by factors like phyosynthetic area, leaf angle and orientation and their exposure to sunlight, light incidents in effective day light period and mineral and water absorption capacities of roots and their transpiration efficiency to leaves. These factors also affects by genotypes and might be possible reasons for differential expression of genes and their enzymes for synthesis of phytochemicals in tested genotypes which were collected from different geographical regions and reported to have differences in morphological features (Rethinam and Sivaraman, 2007). Pichersky and Gang (2000) also stated that the enzymes involved in synthesis of secondary metabolites are products of transcription and translation processes involved in DNA processing in cell system. Similarly, the variations among proximate components and micronutrients in tested 33 genotypes also might be due to genetic factors as reported by Singh et al. (2011c) in traditional vegetables of Andaman and Nicobar Islands.
2.3 Anti-nutrient factors
The anti-nutrients viz., nitrate, phytate, oxalate and saponin contents were estimated in 33 genotypes and results are presented in Table 1. The genotypes showed significant (p<0.05) differences for these compounds and the range for nitrate was observed to be 22.23 to 98.8 mg/100 g, phytate from 185.06 to 967.98 mg/100 g, oxalate from 30.15 to 67.05 mg/100 g) and saponin from 130 mg/100 g to 440 mg/100 g. The highest nitrate was estimated in HD-6, phytate in CHTAP-13, oxalate in ABF-1 and saponin in LH-12. The observed values of anti-nutrients are high but fruits are consumed after thorough processing and in very small quantity as health supplement, hence the actual intake of these compounds remains negligible. However, West et al. (2007) reported low content of phytic acid (<1 g/kg) and oxalic acid (1 g/kg) in raw leaf samples of M. citrifolia from 11 islands throughout French Polynesia.
2.4 DPPH Antioxidant activity
The DPPH antioxidant activity methanol extract of fruits fro 33 genotypes of M. citrifolia was ranged from 49.2% to 88.7%, the highest in FRG-14 and lowest in GAH-1 (Table 1). The other genotypes with high antioxidant activity were identified as HD-6 (88.83 %), HBAY-11A (83.0%), WAND-4 (82.6%), TRA-1 (82.4%), CHLD-17 (79.2%) and TRA-2 (76.8%). The antioxidant activity of plant extracts is associated with compounds, such as flavones, flavonols and proanthocyanidins (Skerget et al., 2005), carotenoids and polyphenol in plant systems (Singh et al., 2011c). The present study also observed good correlation for antioxidant activity with carotenoids and flavonoids while positive correlation with phenol, tannin and ascorbic acid. The findings are in conformity with the previous reports of Katalinic et al. (2006) and Singh et al. (Singh et al., 2011a; Singh et al., 2011c).
|
.png)
Figure 2 Variability in Morinda citrifolia germplasm for proximate composition and micronutrients content in fruits at maturity stage
|
|
.png)
Figure 3 Variation in fruit characters in Morinda citrifolia germplasm
|
|
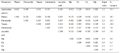
Table 2 Correlation analysis between antioxidant and phytochemicals and micronutrients in 33 genotypes of M. citrifolia L.
|
2.5 Proximate composition
The results for extent of diversity in proximate components in 33 genotypes of M. citrifolia are presented in Figure 2. The highest juice recovery was observed in HD-6A (65.5%) while lowest in MEM-1 (22.41%). Crude fiber content was ranging from 7.62% (BRJ-19) to 9.97% (MEM-1). The fruits of M. citrifolia were found to be rich in protein (2.9-6.0%) (Figure 2). Fat content in fruit samples was observed to be very poor (0.09-0.24%). The total soluble solids (6.0° Brix in SPG-2 to 9.8° Brix in ABH-1) and titrable acidity (0.14% in AHD-1 to 0.41% in CHLD-17) showed significant (p<0.05) variations. Carbohydrate content in fresh fruits was low as it ranged from 224.16 mg/100 g (MANJ-1) to 930.4 mg/100 g (LH-1). The observed values are in the range of earlier findings of Satwadhar et al. ( 2011) and the identified genotypes with high TSS and acidity have good promise in herbal processing industry.
2.6 Micronutrients
The results of micronutrient estimation in 33 genotypes of M. citrifolia are presented in Figure 3. Mn content was observed to highest in SPG-2 (284.2 ppm) while lowest in ABH-1 (4.6 ppm). M. citrifolia genotypes showed great variation for calcium content in fruits which ranged from 51.1 ppm (GAH-1) to 6815.6 ppm (MBAY-16). Similarly, the genotypes showed significant (p<0.05) variation for calcium content which ranged from 16.3 ppm (GAH-2) to 1131.3 ppm (AHD-1). Copper content in genotypes ranged from 2.9 ppm to 17.2 ppm, highest in PBAY-7 and minimum in MHP-19. The micronutrients also play key role in health benefiting properties of plant extracts, hence, genotypes identified could be useful for preparation of nutrient dense herbal supplements.
2.7 Correlation and regression studies
The results for correlation and regression analysis of phytochemical contents in M. citrifolia germplasm are presented in Table 2. The DPPH antioxidant activity of fruits showed good correlation with carotenoids (r=0.335) and flavonoids (r=0.249). Interestingly, the present study revealed strong correlation between antioxidant activity and micronutrients such as Cu (r=0.953), Mn (r=0.953) and Mg (r=0.582). However, Ca content also showed good correlation with antioxidant activity (r=0.220). Among phytochemicals, the carotenoids content in M. citrifolia germplasm showed strong correlation with ascorbic acid (r=0.973), tannin (r=0.598), flavonoids (r=0.691) and phenol (r=0.598). The correlation analysis between phytochemicals and micronutrients revealed strong positive correlation viz., flavonoids and Mn (r=0.902); tannin and Cu (r=0.916) carotenoids and Mg (r=0.553). Strong correlation between micronutrients and antioxidant activity in M. citrifolia germplasm which might be due to role of micronutrients as co-factors in free radical scavenging reactions or their role in synthesis of anti-oxidative phytochemicals. The study identified GAH-1, CHTAP-13, TRA1 and TRA2 as potential genotypes for higher recovery of phytochemicals in from fruits fractions.
3 Discussion
The superior genotypes with higher contents of phytochemicals and micronutrients and lower values for anti-nutritional factors were identified in present study. These genotypes can be promoted for commercial cultivation rather than local landraces for higher recovery of bioactives in fruit juice of M. citrifolia. Four of the 33 genotypes identified as superior for fruit traits and phyto-nutrients were identified as CIARI Samridhi, CIARI Sampada, CIARI Sanjivini and CIARI Rakshak for commercial cultivation. Hence, the information will be further useful for breeding of Noni genotypes rich in complex viz viz individual compounds for industrial promotion of this recently domesticated crop. The study also provided base genetic materials for study the genetics of phytochemicals in Noni for devising appropriate approaches to study the system biology of this pharmaceutically wonder plant.
4 Materials and Methods
The fresh, mature fruits were collected from 33 genotypes of M. citrifolia, grown in Noni Germplasm Block, Central Agricultural Research Institute, Port Blair, Andaman and Nicobar Islands, India (11°36'N; 92°42'E). The samples were divided into three sets, one for observation on fruits traits and second for phytochemical and micronutrient analysis. For phytochemical analysis, 2 g fresh fruit pieces from each accession was grounded well with 10 ml methanol using mortar and pestle and filtered using Whatman No.1 filter paper. The collected sample-solvent mixtures were centrifuged at 8000 rpm for 10 min and concentrated by rotary evaporator (CyperLab, USA). The methanol:formic acid:water (70: 2: 28) and acetone were used as a solvent for anthocyanin and carotenoid extraction, respectively. The samples were preserved at -20℃ for further analysis.
4.1 Observation of fruit traits
Fruit traits viz., fruit length, width and weight were taken using standard measurement scales. Fruits were dissected into pulp and seed and both weighed using electronic balance. Percentage values of pulp and seed contents were calculated with reference to their share in whole fruit weight.
4.2 Phytochemical estimation
Polyphenol content in 33 genotypes of M. citrifolia was determined by Folin-Ciocalteau method (10%, v/v) (Singleton and Rossi, 1965) with minor modifications. The absorbance was measured at 765 nm using UV-spectrophotometer (Elico SL-164, India) and results were expressed as mg of gallic acid equivalent. Flavonoid content was determined as described by Chang et al. (2002) and expressed as mg/100 g fresh weight (fw). Concentration of anthocyanin was determined by pH differential method with cyanidine-3-glucoside (C3GE) as standard and results were expressed as C3GE mg/100 g fresh weight (Fulecki and Francis, 1968). Ascorbic acid and carotenoids contents in fruit samples were determined by the procedure described by Sadasivam and Manickam (1996). Tannin content was estimated using AOAC method (AOAC, 1995) with tannic acid as standard and expressed as mg/100 g fw.
4.3 DPPH antioxidant activity
The antioxidant activity of fruit extracts was analysed using the DPPH (2, 2-diphenyl-1-picrylhydrazyl) method as described by Singh et al. ( 2011c) for underutilized fruits. Briefly, 0.1 ml sample was mixed with 3 ml of 0.001 M DPPH in methanol. Absorbance at 517 nm was observed using UV–visible spectrophotometer after 30 min of incubation. The % inhibition was calculated using the standard formula: [(Ao–Ae)/Ao]*100 (Ao-absorbance for blank; Ae-absorbance for sample).
4.4 Proximate composition
Carbohydrate, protein, fat contents of samples were estimated using standard protocols described by Sadasivam and Manickam (1996) where crude protein in samples was determined by formula, CP (%)=N×6.25; where N is total nitrogen in fruits. Fat content determined by Soxhlet extractor apparatus and crude fibre content by acid-base digestion method as described by Hassan et al. (2008). Total soluble solids in the fruits were determined by refractometer (Atago, Japan).
4.5 Anti-nutritional factors
Anti-nutrional compounds such as phytate, nitrate and oxalate were determined as described by Hassan et al. (2008) and saponin content was determined by the AOAC method (1995).
4.6 Micronutrient estimation
Micronutrients such as magnesium, calcium and copper were analysed using Atomic Absorption Spectrophotometer (AAS; Shimandzu AA 6200, Japan). Briefly, a known quantity sample was converted into ash in a muffle furnace and dissolved in deionised water. The samples were read on the AAS after appropriate dilution.
4.7 Statistical analysis
Experimental results were analyzed for Pearson correlation coefficient of phytochemicals with antioxidant activity (DPPH assay) and tested for significance (p<0.05; p<0.01) using WINKS SDA software. The graphical work and linear regression curve were done by Microsoft Excel Software 2007.
Acknowledgement
Authors express thanks for financial support of National Medicinal Plant Board, New Delhi and World Noni Research Foundation, Chennai and the director ICAR-CIARI, Port Blair for laboratory facilities.
AOAC, 1995, Association of Official Methods of Analysis, 16th ed., Washington DC, USA
Balakrishnan N.P., and Ellis J.L., 1996, Andaman and Nicobar Islands, In: Sharma B.D., Hajra P.K., and Sanjappa M. (eds.), Flora of India: Introductory Volume (Part 1), Botanical Survey of India, Calcutta, India, pp.1-538
Chang C.C., Yang M.H., Wen H.M., and Chern J.C., 2002, Estimation of total flavonoid content in propolis by two complementary colorimetric methods, J Food Drug Anal, 10: 178-182
European Commission Scientific Committee of Food, 2002, Opinion of the Scientific Committee on Food of Tahitian Noni Juice, SCF/CS/DOS/18 ADD 2, Belgium
Fulecki T., and Francis F.J., 1968, Quantitative methods for anthocyanin extraction and determination of total anthocyanin in cranberries, J Food Sci, 33: 72-77
Hassan L.G., Dangoggo S.M., Umar K.J., Saidu I., and Folorunsho F.A., 2008, Proximate, minerals and antinutritional factors of Daniellia oliveri seed kernel, Chem Class J., 5: 31-36
Katalinic V., Milos M., Kusilic T., and Jukic M., 2006, Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols, Food Chem., 94: 550-557
McClatchey W., 2002, From Polynesian Healers to Health Food Stores: Changing Perspectives of Morinda citrifolia (Rubiaceae), Integr Cancer Ther, 1(2): 110-120
PMid:14664736
Pichersky E., and Gang D.R., 2000, Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective, Trends Plant Sci Perspect, 5(10): 439-445
Rasoanaivo P., Wright, C.W., Willcox M.L., and Gilbert B., 2011, Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar J., 10(1): S4
Razafimandimbison S.G., McDowell T.D., Halford D.A., and Bremer B., 2009, Origin of the pantropical and nutriceutical Morinda citrifolia L. (Rubiaceae): comments on its distribution range and circumscription, J Biogeogr, 37(3): 520-529
Rethinam P., and Sivaraman K., 2007, Noni (Morinda citrifolia L) the Miracle Fruit -A Holistic Review. Inter J Noni Res, 1(1-2): 4-37
Sadasivam S., and Manikam A., 1996, Hand book of biochemical methods, Wiley Eastern Ltd., Madras, India
Sandeep I.S., Nayak S., and Mohanty S., 2015, Differential effect of soil and environment on metabolic expression of turmeric (Curcuma longa cv. Roma), Indian J Exp Bio, 53: 406-411
Satwadhar P.N., Deshpande H.W., Syed I.H., and Syed K.A., 2011, Nutritional composition and identification of some of the bioactive components in Morinda citrifolia juice, International Journal of Pharmacy and Pharmaceutical Sciences, 3(1): 58-59
Singh D.R., Singh S., Minj D., Anbananthan V., Salim, K.M., Kumari C., and Varghese A., 2012, Diversity of Morinda citrifolia L. in Andaman and Nicobar Islands (India): assessed through morphological and DNA markers, African J Biotech, 11(86): 15214-15225
Singh D.R., Singh S., Salim K.M., and Srivastava R.C., 2011a, Estimation of phytochemicals and antioxidant activity of underutilized fruits of Andaman and Nicobar Islands (India), Int J Food Sci Nutr, 63(4): 46-52
Singh D.R., Srivastava A.K., Srivastava A., and Srivastava R.C., 2011b, Genetic diversity among three Morinda species using RAPD and ISSR markers, Indian J Biotech., 10: 285-293
Singh D.R., Singh S., and Srivastava R.C., 2010, Noni (Morinda citrifolia L.), In Peter K.V. (eds.), Editor Future crops Vol. I, Daya Publishing House, New Delhi, pp.143-171
Singh S., Singh D.R., Banu V.S., and Avinash N., 2014, Functional constituents (micronutrients and phytochemicals) and antioxidant activity of Centella asiatica (L.) Urban leaves. Ind Crops Prod, 61: 115-119
Singh S., Singh D.R., Salim K.M., Srivastava A., Singh L.B., and Srivastava R.C., 2011c, Estimation of proximate composition, micronutrients and phytochemical compounds in traditional vegetables from Andaman and Nicobar Islands, Int J Food Sci Nutr, 62(7): 765-773
PMid: 21615278
Singleton V.L., and Rossi J.A., 1965, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, Am J Enol Vitic, 16: 144-158
Skerget M., Kotnik P., Hadolin M., Hras A., Simonic M., and Knez Z., 2005, Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidants activities, Food Chem., 89: 191-198
USPTO, 2005, Patent Full-Text and Image Database, Patents (Morinda citrifolia)
West B.J., Tani H., Palu A.K., Tolson C.B., and Jensen C.J., 2007, Safety tests and antinutrient analyses of noni (Morinda citrifolia L.) leaf, J Sci Food Agric., 87(14): 2583-2588

 Author
Author  Correspondence author
Correspondence author
.png)
.png)
.png)
.png)



