Research report
Variation and Repeatability of Natural Antibodies Against Keyhole Limpet Hemocyanin of Indigenous Chicken of Kenya 
2 Department of Biochemistry and Molecular Biology, Egerton University P.O. Box 536, 20115 Egerton Kenya
3 Department of Animal Science, Maseno University P.O. Box private Bag, 40105 Maseno Kenya
 Author
Author  Correspondence author
Correspondence author
Genomics and Applied Biology, 2016, Vol. 7, No. 4 doi: 10.5376/gab.2016.07.0004
Received: 08 Jan., 2016 Accepted: 16 Mar., 2016 Published: 21 Oct., 2016
Khobondo J.O, Mwakubambanya. R, Wasike, C.B., and Upreti H.K., 2016, Variation and repeatability of natural antibodies against Keyhole limpet hemocyanin of indigenous chicken of Kenya, Genomics and Applied Biology, 7(3): 1-8 (doi: 10.5376/gab.2016.07.0004)
The immune system is designed to provide protection to the body by combating pathogens. Identifying animals with superior immune responses reduces disease occurrences, increases farm profit and improves product quality and safety. Consequently, there is need to breed disease resistant animals that will eliminate the danger of currently used disease management strategies; drug prophylaxis and vaccination, which are unsafe and ineffective respectively. studies aimed at investigating the mechanisms involved in genetic resistance have been done, however a standardized biological parameter indicative of disease resistance or susceptibility remains elusive. The objectives of the study were to determine presence and variation of IgA, IgG and IgM among indigenous chicken. Estimate repeatability within the indigenous chicken over time of IgA, IgM and IgG natural antibody isotypes against Keyhole limpet hemocyanin. Blood samples from 24 indigenous chickens of the same age and sex were collected four times within three weeks. IgA, IgM and IgG titer values were measured by indirect ELISA from the sera. A mixed model with repeated measures was performed to determine variation and repeatability. All the immunoglubulin isotypes binding KLH in chicken serum were recorded. There was significant difference between isotypes concentrations with IgM and IgA being the lowest and highest titre values, respectively. Repeatability was 0.68, 0.99 and 0.99 for IgM, IgG and IgA, respectively. The isotypes were detectable and variable in serum of indigenous chicken and consistently and repeatedly measurable in blood serum. This finding may lay the basis for genetic improvement of immune response in the indigenous chicken.
1 Introduction
Infectious diseases are of major importance to livestock breeders due to cost, potential zoonotic threats, animal welfare issues, and threats arising from breakdown of currently used diseases’ control strategies. In the tropics, predominant diseases vary between production systems which are mostly at risk. Endemic infectious diseases are also problematic because traditional disease control strategies are failing. In such cases, alternative or complementary sustainable control strategies such as breeding programs to increase host resistance to infection (or disease) are required. For these reasons, disease resistance is now one of the major targets of genetic studies in livestock aimed at conferring heritable ability to the animal to evade or withstand infection as a result of enhanced innate and acquired immunity (Parmentier et al., 2004).
Innate immunity as the first line of defense plays an important role in preventing or combating infection (Ternynck and Avrameas, 1986). Amongst the components of innate immunity are natural antibodies (Nab) of the humoral arm which play an important role of combating diseases, augmenting immune response and immunosenescence (Baumgarth et al., 2005; Elluru et al., 2008). Natural antibodies are present in nonimmunized cattle (Van Knegsel et al., 2007), humans (Ehrestein et al., 2010), rats, rabbits, python (Ujvari et al., 2011) and poultry (Sun et al., 2011). These antibodies do arise independent of known antigenic stimulation and do not require prior antigenic stimulation. They are mostly polyreactive, polyspecific (Dimitrov et al., 2005), with low binding affinity (Casali and Notkins, 1989) and generally encoded by the ummutated V genes in germline configuration (Lutz et al., 2007). The role of Nab involves provision of a barrier to infection by providing a pre-existing antibody reactivity thus allowing animals to recognize the invading pathogen prior to adaptive immunity (Chou et al., 2008).
Most of Nab binds pathogen-associated molecular patterns (PAMPs) that are conserved along different genera and this serve as targets for identification of microbes by the innate immune system (Kohler et al., 2003). Important PAMPS are lipopolysaccharide (LPS) present on gram-negative entero-bacteria, such as E. coli or Salmonella spp.; lipoteichoic acid (LTA) present on gram-positive bacteria, such as S. aureus; or peptidoglycan (PGN) present on gram-negative and gram-positive bacteria (Ploegaert et al., 2010). Besides the PAMPs, Nab do bind many solubilized extracts of liver, kidneys, stomach, muscle, thymus, lungs, nuclear, tumors and red blood cells components prepared in the presence of Sodium dodecyl sulfate (SDS) (Madi et al., 2010).
Natural antibodies can be categorized into two classes: the first class are directed against self antigens, are called natural auto antibodies (Naab) or cryptic, hidden, masked, latent or silent Nab (Cheng and Chamley, 2008; Khobondo et al., 2015a). They inactivate cytokines, clear obsolete or damaged cells and metabolic waste and are believed to play part in surveillance or homeostasis maintenance (Lutz et al., 2007). The second class are the overt Nabs, which are readily detected in unfractionated untreated normal sera. These Nabs bind antigen that an individual has never encountered before such as keyhole limpet hemocyanin (Bergstra et al., 2010).The Keyhole limphet hemocyanin is a protein antigen from megathura cranulata, a deep sea organism that is assumed never to have had prior encounter with terrestrial animals. Therefore any immune response against it will be innate in nature (Parmentier et al., 2004). Nab can thus be tested as a parameter for immune response and genetic variation estimation.
It is a prerequisite that, for a parameter of disease resistance to be acceptable in genetic studies, it should be variable to justify selection. Furthermore, there is no standard biological molecule so far to measure disease resistance or tolerance. Several disease parameters have been investigated to measure immunity and disease resistance. These include cytokines; tumor necrosis factor (TNF) and interferon (IFN), cellular components (eg B, T and NK cells) and Reactive Oxygen Sulphite (ROS) production by phagocytic cells in blood and milk from healthy cows (Ploegaert et al., 2010). A study by Ploegaert et al. (2010) reported high repeatability and significant variation of cytokines as well as reasonable repeatability of Nabs and milk components of cows. Other than variation, a trait should be repeatable to be sampled once under the same environmental conditions. Repeatability of a parameter is important to assess whether a single test on single sample collection is adequate to make inferences or reproducible thus reducing the cost of taking repeated measures. The value of repeatability can be useful in estimating the upper limit of heritability.
Overt Nabs could be the antibodies of choice for exploring disease resistance parameter and future association with diseases in IC and other animals. Therefore the objectives of this study were a) to determine the presence and variation of Nab isotypes IgA, IgG and IgM binding KLH in clinically healthy indigenous chicken (IC) and b) to estimate repeatability of IgA, IgG and IgM Nab titres binding KLH of IC sampled four times in three weeks. The research findings give useful insight into genetic basis of Nabs and prospects of selective breeding for disease resistance or association in IC.
2 Material and Methods
2.1 Experimental population
The base population of chicken used in this study were established through collection of chicken and eggs from unselected, random mating population of IC from the rural farmers of Kakamega, Bondo, West Pokot, Narok, TaitaTaveta, Lamu and Bomet counties in Kenya thus called ecotypes. The counties were chosen because there has been minimum genetic dilution of the IC by introduction of exotic chicken. From these chicken and eggs, a population of IC was established on station at Egerton University.
From the established population, eggs were simultaneously incubated but separated according to ecotype within incubator. At hatching, each chick was wing tagged with an identification number. Brooding was from hatching to 6 weeks. Brooding of chicks from each ecotype was separated in deep litter brooders using infra-red electric bulbs. The population density was 12birds/m2. At the beginning of the 7th week, chicks were transferred to randomly selected deep litter rearing pens within the same house. Sex was determined by phenotypic appearance.
2.2 Health and disease management
The chicken received routine vaccinations against Marek’s disease (d 1), New Castle disease (NCD; wk 2, 6, 12, 15), infectious bronchitis (d1, wk 2, 10, 12, 15), infectious bursal disease (wk 3, 15) and fowl pox (wk 15).
2.3 Overt natural antibodies isotype assays
Blood samples (2 ml in EDTA) from 24 IC for variation and repeatability studies was drawn from the wing vein of each bird and serum separated by centrifugation at 2000rpm for 10 minutes. These birds were all female, same age, genetic background and under same management. Isotype specific IgA, IgM and IgG antibody titres to keyhole limpet hemocyanin (KLH) in serum from the IC were determined by indirect enzyme-linked immunosorbent assay (ELISA) as described by (Khobondo et al., 2015c). Briefly, 96 well plates were coated with 2μg/ml KLH (MP Biomedicals Inc., Aurora, OH) and incubated overnight at 4°C. The plates were washed using a washing/dilution buffer (phosphate buffered saline (PBS) containing 0.05% Tween) and incubated for 1.5 hours at 25°C with IC serum diluted 1:10 in dilution fluid. The plates were washed using washing buffer to remove unbound serum. To detect IgA, IgM and IgG antibodies binding to KLH, a secondary 1:20,000 diluted affinity purified goat anti-chicken IgM (Fc specific), conjugated with horseradish peroxidase (GACh/IgM (Fc) /PO) antibody, or 1:20,000 diluted whole rabbit anti-chicken IgG (heavy and light chains) conjugated with horseradish peroxidase (RACh/IgG(H+L)/PO) antibody or 1:20,000 diluted affinity purified goat anti chicken IgA (Fc specific) conjugated with horseradish peroxidase (GACh/IgA (Fc) /PO) (Nordic Immunological Laboratories, Eindhoven, The Netherlands ) was added and incubated for 1.5 hours at 25°C. The plates were washed again and 100 μL substrate-buffer (containing aqua dest, 10% tetramethylbenzidine-buffer and 1.33% tetramethylbenzidine) was added in each well and incubated for 10 minutes at room temperature. The reaction was stopped with 1.25 M H2SO4. Absorbance levels were measured with a spectrophotometer (mrc Scientific Instrument-UT- 6100, Israel) at 450 nm.
2.4 Statistical analysis
Descriptive statistics were used to explore the data and linear model in SAS (SAS Institute Inc., Cary;Version 9.1) used for initial analysis. Because Isotype of antibodies (IgA, IgG, IgM) were treated as different parameters, further analysis were performed using separate isotypes. Variation was estimated using the following mixed model;
Yij = µ + timei+ ICj +eij
Where Yij is the Nab titre (IgM or IgG or IgA) of ICj at timei, µ is the common mean. Time is the fixed effect of time of measurement i (i = 1, 2, 3, 4). IC is the random effect of the ICj (j = 1...24; normal, independent and identically distributed (0 = σ2IC) and eij is the random residual (normal, independent and identically distributed).
Repeated measures analyses was performed using PROC MIXED of SAS (SAS Institute Inc., Cary;Version 9.1). Covariance structure used was compound symmetry (αI= δ2|i=j). Model assumptions regarding normality were evaluated by examining whether skewedness and kurtosis were close to 0 and whether a probability plot did not show deviation from a straight line.
Repeatability (r) along time and within IC was calculated as:
where δ2 IC is the variance among IC and δ2e is the residual variance.
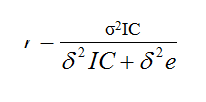
3 Result
3.1 Presence and variation of natural antibodies against KLH titres in Serum of IC
Natural antibodies binding KLH were detected for all IgA, IgG and IgM isotypes in IC serum. The analysis of variance showed significant difference (p˂0.0001) with time of sampling being the main source of variation. IgM concentration/titre value had the highest means but with minimum variance and standard error of the mean. The IgG had the highest variance and standard error of the mean with moderate means (Table 1).
|
Table 1 The Means and standard deviations of Isotype on the levels of Nabs titres of IC against KLH antigens. The IgM had higher Nabs titres with lower standard error (SE) |
3.2 Repeatability of natural antibodies titers
Natural antibodies (IgA, IgG and IgM) titers of IC were determined at 4 points to assess their repeatability. The estimated repeatability for IgA, IgG and IgM was 0.99, 0.68 and 0.99 respectively with IgG isotype showing highest variance (Table 2).
|
Table 2 Natural IgA, IgM and IgG isotypes antibodies titers binding keyhole limpet hemocyanin in blood serum of IC; overall variance, variance within and among IC, significance of variation and estimates of repeatability |
4 Discussion
The lack of effective control measures for infectious diseases (management, vaccination and prophylaxis) in livestock not only causes significant economic losses but may also endanger human health through zoonosis, compromise animal welfare and food security (Stear et al. 2012). The use of genetic selection of animal for traits of resistance to infection has been presented as the “ultimate tool in sustainable disease control” (Waller, 2006; Khobondo et al. 2014; Khobondo et al. 15b). Disease resistance and immune response is a complex trait polygenically expressed with several immune proteins. Due to this complexity, the most important and rate limiting challenge for disease genetic studies is likely to be obtaining suitable phenotypes (immune parameter). In goats and sheep for example, selection is based on the phenotyping of relevant traits such as zootechnical performance, Fecal Egg Count (FEC), and measures of anaemia and blood eosinophilia under conditions of either natural or experimental nematode infection (Mandonnet et al. 2014). In cattle, several immune parameters have been reported to be potentially related to susceptibility or resistance to various diseases (Thompson-Csrispi et al. 2013). These include soluble mediators like Nabs, cytokines, antimicrobial peptides and complement proteins, and cellular components like B, T and NK cells, y-T cells and granulocytes (Ploegaert et al. 2008). Therefore, choice of immune parameter for disease resistance study is critical (Biochard and Brochard, 2012). For example, specificity of acquired immunity is limiting to other pathogen except the one the animal has been primed with before. Therefore, natural antibodies could be promising since it is polyreactive and non specific despite being slow to combat pathogens.
In consistency with other studies (Ploegaert et al. 2010; Ujvari et al. 2011) the current study reported the presence of Nabs isotypes IgA, IgG and IgM in IC’ serum. This study again confirms that the IgM Nabs isotype is the major isotype. In previous studies, IgM has accounted for most of the B cell repertoire in the fetus and neonate, and possibly play a major role in the development and physiology of the mammals B cell repertoire (Boes, 2000). Most Nabs of the IgM isotype class are present in vertebrates, but IgG and IgA Nabs are also present in higher vertebrates (Boes, 2000). The Nabs of IgM isotype are mostly produced by CD5+B cells in the peritoneal and intestinal cavity but also CD5- B cells (Zhou et al., 2004). The other isotypes namely IgA, IgE, IgD and IgG do arise from IgM class switching and this phenomenon could justify the higher titers of IgM as compared to IgG and IgA in this study. The choice of Nab in this study is pegged on the fact that it is the natural arm of humoral immune response, polyreactive and nonspecific in nature (Parmentier et al. 2004). For a parameter to be used as a potential measure for genetic disease resistance, it should be variable among individual animals. In genetics, lack of variability of a parameter show that the animals are naturally the same with respect to immune parameter thus limiting selection (Khobondo et al. 2014). In the current study Nabs binding KLH were found to be significantly variable among IC and could be exploited in selection for immunoglobulins titre values a proxy for immune competence. Star et al. (2007) reported Nabs binding KLH to be variable in layers, the same has been confirmed in other animals like cattle (Ploegaert et al. 2010) and also in this study using IC. The repertoire and levels of Nabs are dependent on several factors, amongst them is the environment (Kachamakova et al. 2006), genetic background (Sun et al. 2011) and age (Parmentier et al. 2008; Ujvari et al. 2011). Evidence from various studies showed Nabs to be genetically controlled (Gonzalez et al. 1989; Sun et al. 2011) and the same is true in the current study.
Repeatability of IgA, IgG and IgM titres in the context of this study was assessed as well. The purpose was to ascertain whether a single test on one sample collection was enough for inferences thus reducing the cost of doing multiple measures. Alternatively repeatability could imply how reproducible or the same a parameter (IgA, IgG or IgM) were under the same environment and conditions of experiment. This genetic parameter have been useful in estimating the upper limit of heritability as well. In deed the repeatability estimate for IgM (0.68), IgA (0.99) and IgG (0.99) were moderate to high. The lower repeatability of IgM experienced in this study was partially expected. The IgM is the primary or precursor of other antibodies. It could therefore be a more stable isotype with time and in different or same environment, a deviation from this finding. Thus a higher repeatability of IgM than other isotypes could be expected under this school of thought. Alternatively, the IgM could represent the continuous presence of randomly produced antibodies that fit both exogenous and autologous plethora of antigens. The formation of antibody idiotypes is a random process through recombination or conversion; this could be true with IgM justifying the class switching role hence lower repeatability evidenced. The low repeatability might also imply the high turnover that this isotype undergo within a very short duration. It therefore means it is an isotype on transit and very unstable depending on environmental stimuli and antigen specificity. The genetic interpretation of the moderate repeatability estimate would imply a large variance between IC (genetic) as compared to variance within time (environment). This mean that genetic component plays important role in variation observed than non-genetic factors with regard to IgM antibody titre and repertoires.
The higher repeatability for IgG (0.99) and IgA (0.99) as compared to IgM realized in this study is however justifiable. This higher estimates could imply lack of plasticity (ie stability) of this isotypes with time and in different or same environment with respect to KLH (antigen in question), hence the low and high variances within the sampling period and between IC respectively. The large variance estimate is conferred by the IC (genetic) but not time period (environment). It has to be kept in mind that this study is the first to determine repeatability of the anitibody titres in IC and further comparison with other related studies is limiting. The moderate to high repeatability for both isotypes in this study therefore affirms that a single measurement can be used to infer reference to Nabs titers of IC along time under same situations/environment with KLH specificity. Thus the result can be reproduced under same management and conditions of the study and would infer to the ability of what is observed being reproduced in the next generation under similar management. This eliminates the need to make several samples from the same IC over time. But for precision, the age of measurement or sampling should be indicated. The high repeatability found in this study, could enhance animal welfare especially on invasive blood sampling procedures and reduce cost especially to immunological assays that are very expensive.
In plasma of mature cows, however, repeatability of total Nabs against LPS, LTA and PGN was estimated to be 0.79, 0.80 and 0.93 respectively, and 0.60 for Nab binding KLH (Ploegaert et al. 2010). In milk, the repeatability estimates of nabs for LPS, LTA, PGN and KLH were 0.74, 0.81, 0.84 and 0.85 respectively (Ploegaert et al., 2010). In another study in cows, repeatability estimates of daily milk, fat and protein yields in cows ranged from 0.63 to 0.83 at different stage of lactation (Vanconcelos et al. 2004). These estimates although comparable to the IC study could not be legitimately used to expound on one another. This is because chicken and cattle are genetically distinct species and possess different genetical physiology. Moreso, the parameter of repeatability estimates are different with respect to samples and secondary antibody used, duration of experiment and experimental conditions. Generally, binding of serum Nabs to KLH a model antigen that teresterial animal has never encountered might reflect immune competency. Therefore well designed studies are required to test this hypothesis. The polygenic and nonspecific nature of Nabs in the host would probably deal with a plethora of epitopes in pathogens and may cooperate with other immune cells and compliment other immune proteins in combating diseases.
5 Conclusion
Natural antibodies isotypes IgA, IgG and IgM binding KLH were detected in blood serum of IC. The Nabs titers were variable within the IC and highly repeatable for IgA, IgG and IgM. Both isotypes can be used for further studies to explore association with disease resistance.
Acknowledgements
The authors are grateful to the Indigenous Chicken Improvement Programme (InCIP), Innovations For Livestock Industry (iLINOVA) Projects in Kenya financed by the European Commission.
Avrameas S. (1991) Natural autoantibodies: from 'horror autotoxicus' to 'gnothiseauton'. Immunol. Today., 12, 154–159.
http://dx.doi.org/10.1016/S0167-5699(05)80045-3
http://dx.doi.org/10.1016/0167-5699(91)90080-D
Baumgarth N., Tung J.W., Herzenberg L.A. (2005) Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol., 26, 347–362.
http://dx.doi.org/10.1007/s00281-004-0182-2
PMid:15633017
Bergstra T.J., Smeets K., Nieuwland M.G.B., Parmentier H.K. (2010) In vivo and vitro post translation polymorphism of chicken natural auto-antibodies. Dev. Comp. Immunol., 34, 821–827.
http://dx.doi.org/10.1016/j.dci.2010.03.002
PMid:20230852
Boes M. (2000) Role of natural and immune IgM antibodies in immune responses. Mol. Immunol., 37, 1141–1149.
http://dx.doi.org/10.1016/S0161-5890(01)00025-6
Boichard D., Brochard M. (2012) New phenotypes for new breeding goals in dairy cattle. Anim., 6:544–550
http://dx.doi.org/10.1017/S1751731112000018
PMid:22436268
Casali P., Notkins A.L. (1989) CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol. Today., 10, 364–368.
http://dx.doi.org/10.1016/0167-5699(89)90268-5
Cheng H.M., Chamley L. (2008) Cryptic natural autoantibodies and co-potentiators. Autoimmun. Rev., 7, 431–434.
http://dx.doi.org/10.1016/j.autrev.2008.03.011
PMid:18558357
Dimitrov J.D., Ivanovska N.D., Lacroix-Desmazes S., Doltchinkova V.R., Kaveri S.V., Vassilev T.L. (2005) Ferrous ions reactive oxygen species increase antigen binding and anti-inflammatory activities of immonuglobulin G. J. Biol. Chem., 281, 439–446.
http://dx.doi.org/10.1074/jbc.M509190200
PMid:16246843
Elluru S.R., Vani J., Delignat S., Bloch M.F., Lacroix-Desmazes S., Kazatchkine M.D., Kaveri S.V., Bayry J. (2008) Modulation of human dendritic cell maturation and function by natural IgG antibodies. Autoimmun. Rev., 7, 487–490
http://dx.doi.org/10.1016/j.autrev.2008.04.014
PMid:18558367
Gonzalez R., Charlemagne J., Mahana W., Avrameas S. (1998) Specificities of natural serum antibodies present in phylogenetically distinct fish species. Immunol., 63, 31–36
Kachamakova N.M., Irnazarow I., Parmentier H.K., Savelkoul H.F.J., Pilarczyk A., Iegertjes G.F. (2006) Genetic differences in natural antibody levels in common carp (Cyprinus carpio L). Fish & Shellfish Immunol., 21, 404–413
http://dx.doi.org/10.1016/j.fsi.2006.01.005
PMid:16545579
Khobondo J. O., Mike G.B., Nieuwland, Laura E Webb, Eddie AM Bokkers and Henk K Parmentier (2015a) Natural (auto) antibodies in IC are affected by age and diet. Vet. Quart., DOI: 101080/0165217620151009657
Khobondo J. O., Muasya T. K., Miyumo S., Okeno T. O., Wasike C.B., Mwakubambanya R., Kingori A.M., Kahi A. K. (2015b) Genetic and nutrition development of indigenous chicken in Africa. Livestock Research for Rural Development Volume 27, Article #159 Retrieved from htp://wwwlrrdorg/lrrd27/8/khob27159html
Khobondo J. O, Ogore P. B, Atela J. A, Onjoro P. S, Ondiek J. O and Kahi A. K (2015c): The effects of dietary probiotics on natural IgM antibody titres of Kenyan indigenous chicken. Livestock Research for Rural Development. Volume 27, Article #230. from http://www.lrrd.org/lrrd27/11/khob27230.html
Khobondo J.O., Okeno T.O., Lihare G.O., Wasike C.B., Kahi A.K. (2014) The past, present and future genetic improvement of indigenous chicken of Kenya. Animal Genetic Resources, 55, 125–135
http://dx.doi.org/10.1017/S2078633614000332
Kohler H., Bayry J., Nicoletti A., Kaveri S.V. (2003) Natural autoantibodies as tools to predict the outcome of immune response?. Scand. J. Immunol., 58, 285–289
http://dx.doi.org/10.1046/j.1365-3083.2003.01314.x
PMid:12950673
Lutz H.U., Binder C.J., Kaveri S.V. (2009) Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol., 30, 43–51
http://dx.doi.org/10.1016/j.it.2008.10.002
PMid:19058756
Lutz H.U. (2007) Homeostatic roles of naturally occurring antibodies: an overview. J. Autoimmunol., 29, 287–294
http://dx.doi.org/10.1016/j.jaut.2007.07.007
PMid:17826952
Mandonnet N., Mahieu M., Alexandre G., Gunia M., Bambou J.C. (2014) Genetic Resistance to Parasites in Small Ruminants: from Knowledge to Implementation in the Tropics Proceedings, 10 th World Congress of Genetics Applied to Livestock Production
Parmentier H.K., Klompen A.L., De Vries-Reilingh G., Lammers A. (2008) Effect of concurrent intratracheal lipopolysaccharide and human serum albumin challenge on primary and secondary antibody responses in poultry. Vaccine, 26, 5510–5520
http://dx.doi.org/10.1016/j.vaccine.2008.07.053
PMid:18694797
Parmentier H.K., Lammers A., Hoekman J.J., De Vries-Reilingh G., Zaanen I.T.A., Savelkoul H.F.J. (2004) Different levels of natural antibodies in chickens divergently selected for specific antibody responses Dev.Comp. Immunol., 28, 39–49
http://dx.doi.org/10.1016/S0145-305X(03)00087-9
Ploegaert T.C., De Vries-Reilingh G., Nieuwland M.G., Lammers A., Savelkoul H.F., Parmentier H.K. (2007) Intratracheally administered pathogen-associated molecular patterns affect antibody responses of poultry. Poult. Sci., 86, 1667–1676
http://dx.doi.org/10.1093/ps/86.8.1667
PMid:17626812
Ploegaert T.C.W., Tijhaad E., Lamd T.J.G.M., Taverne-Thielea A., van der Poelb J.J., van Arendonk J.A.M., Savelkoul H.F.J., Parmentier H.K. (2011) Natural antibodies in bovine milk and blood plasma: Variability among cows, repeatability within cows, and relation between milk and plasma titers .Vet. Immunol. Immunopathol, doi:101016/jvetimm201107008
http://dx.doi.org/10.1016/j.vetimm.2011.07.008
SAS Institute (1990) SASR User's Guide: Statistics Version 6 Edition SAS Institute Inc, Cary, NC
Stear M. J., Nikbakht G., Matthews L., Jonsson N.N. (2012) Breeding for disease resistance in livestock and fish. CAB Reviews: Perspectives in Agric. Vet. Sci. Nut. Natural Resources, 7, 1–10
Sun Y., Parmentier K.K., Frankena K., van der Poel J.J. (2011) Natural antibody isotypes as predictors of survival in laying hens. Poultr. Sci., 90, 2263- 2274
http://dx.doi.org/10.3382/ps.2011-01613
PMid:21934009
Ternynck T., Avrameas S. (1986) Murine natural monoclonal autoantibodies: a study of their polyspecificities and their affinities. Immunol. Rev., 94, 99–112
http://dx.doi.org/10.1111/j.1600-065x.1986.tb01166.x
Thompson-Crispi K., Miglior F., Mallard B. (2013) Incidence rates of clinical mastitis among Canadian Holsteins classified as high, average, or low immune responders. Clin Vaccine Immunol., 20,1 06–112
http://dx.doi.org/10.1128/cvi.00494-12
Thompson-Crispi K.A., Miglior F., Mallard B.A. (2013) Genetic parameters for natural antibodies and associations with specific antibody and mastitis in Canadian Holsteins. J. Dairy Sci., 96, (6), 3965–3972.
http://dx.doi.org/10.3168/jds.2012-5919
PMid:23587396
Thompson-Crispi K.A., Sewalem A., Miglior F., Mallard B. (2012) Genetic parameters of adaptive immune response traits in Canadian Holsteins. J. Dairy Sci., 95, 401–409
http://dx.doi.org/10.3168/jds.2011-4452
PMid:22192219
Ujvari B., Madsen T. (2006) Do natural antibodies compensate for humoral immunosenescence in tropical pythons? Funct. Ecol., 25, 813–817
http://dx.doi.org/10.1111/j.1365-2435.2011.01860.x
Van Knegsel A.T., de VriesReilingh G., Meulenberg S., van den Brand H., Dijkstra J., Kemp B., Parmentier H.K. (2007) Natural antibodies related to energy balance in early lactation dairy cows. J. Dairy. Sci., 90, 5490–5498
http://dx.doi.org/10.3168/jds.2007-0289
PMid:18024740
Waller P.J. (2006) From discovery to development: current industry perspectives for the development of novel methods of helminth control in livestock. Vet. Parasitol. 139, 1-14
http://dx.doi.org/10.1016/j.vetpar.2006.02.036
Zhou Z.H., Notkins A.L. (2004) Polyreactive antigen-binding B (PAB+) cells are widely distributed and the PAB+ population consist of both B-1+ and B-1- phenotypes. Clin. Exp.l Immunol., 137, 88–100
. PDF(0KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Joel Khobondo
. Ramadhan Mwakubambanya
. Chrilukovian Wasike
. Alexander Kahi
Related articles
. Indigenous chicken
. Repeatability
. Natural antibodies
. Variation
Tools
. Email to a friend
. Post a comment
.png)
.png)


