2 Department of Biotechnology, University of Agricultural Sciences, GKVK, Bangaluru, 560065, India
3 Biochemistry Division, Department of Chemistry, Central College Campus, Bangalore University, Bangaluru, 560001, India
4 Lilac Insight Pvt Ltd., Ambeience court, Vashi, Navi Mumbai, 400705, India
5 School of Biotechnology, SKUAST-J, Chatha, Jammu-180009, India
 Author
Author  Correspondence author
Correspondence author
Genomics and Applied Biology, 2012, Vol. 3, No. 2 doi: 10.5376/gab.2012.03.0002
Received: 09 Jul., 2012 Accepted: 31 Jul., 2012 Published: 10 Aug., 2012
Chumki et al., 2012, Exploring plant proteinase inhibitors, Genomics and Applied Biology, 2012, Vol.3 No.2 8-21 (doi: 10.3969/gab.2012.03.0002)
Proteinase Inhibitors (PIs) are small, natural antagonists of proteinases and present in all life forms. PIs are widely present in plants and often found in storage organs. They are known to be inducible in plants by injuries, such as insect damage. PIs have enormous diversity of function through regulation of target proteinases. Various plant sources have been explored for isolating PIs and broad-spectrum of biological activities have been elucidated. A range of strategies have been attempted to improve effectiveness of proteinaseinhibitors as antimetabolites towards insects, bacteria and fungi. Much emphasis is yet to be given to address the health benefits of the PIs and implementing it in the most available forms throughout.
Plants are continuously exposed to insect pest and pathogen attacks during their life cycle. Like animals, plants cannot move away from an endangering environment nor do they possess a characteristic immune system. Each plant species or cultivars have developed diverse defense mechanism, which includes both specific and general defense responses. Herbivorous insects, mites and nematodes are major contributors to yield loss either directly through consumption of plant biomass or indirectly as vectors/facilitators of pathogen infection. Consequently, introgression of insect-pest resistance into crops has become one of the major priorities for plant breeders. Resistant cultivars reduce dependence on insecticides and need for crop rotation, provide economic benefits without compromising the environmental stewardship. Studies on host plant resistance reveal that plants have evolved natural defense strategies against pests, including the production of compounds that contribute directly or indirectly, protection against herbivore invasion. The best-known plant substances supposedly involved in defense mechanisms against phytophagous insects are ribosome-inactivating proteins (RIPs), protease inhibitors (PIs), amylase inhibitors (áAl-I) and lectins. Insects have developed several strategies to overcome plant defense barriers, allowing them to feed, grow and reproduce on their host plants. However, insects possess a powerful assemblage of enzymes that constitute their defense against chemical toxicant (Ryan, 1990; Smith and Boyko, 2006).The major digestive proteases present in herbivorous insect gut are serine, cysteine, and aspartyl proteases. Inhibitors of these proteinases have been identified in plants that inhibit gut proteolytic activity, and adversely affect the growth and development of insect pests. Studies reveal that PIs are induced under various stress conditions such as insect attack, mechanical wounding, pathogen attack and exposure to UV. Role of PIs in plants as natural defense has greatly stimulated thinking towards enhancing the plant defense against insect pests through genetic engineering. The strategy to improve plant defenses against insect pests is being tried using serine proteinase inhibitors. Evolutionarily during plant–insect interactions, insects have adapted to the PIs of their host plants. Therefore, non-host plants were also evaluated and subsequently used for transferring these traits into commercial cultivars.
PIs have broad-spectrum of biological activity, which includes suppression of pathogenic nematodes (Williamson and Hussey, 1996), inhibition of spore germination and mycelium growth (Dunaevskii et al., 1997) and hampering the growth of pathogenic fungi (Joshi et al., 1998; Bhattacharjee and Prasad, 2005). In addition, PIs also contribute to better and enhanced nutritional quality of grains, as they are rich in cysteine and lysine (Ryan, 1989, Bhattacharjee et al., 2006). PIs are specific for each of four mechanistic classes of proteolytic enzymes, classified as serine, cysteine, aspartic and metallo-protease based on the active amino acid in their reaction center. In general, PIs are competitive inhibitors, bind to the active site of the enzyme. For instance serine PIs bind to serine proteases, which include trypsin, chymotrypsin, elastase, subtilisin and thrombin. Serpins (serine protease inhibitors or classified inhibitor family I4) are the largest and most broadly distributed superfamily of protease inhibitors (Rawlings et al., 2004). Serpin-like genes have been identified in animals, plants, bacteria, and some viruses (Gettins, 2002). Most serpins are irreversible inhibitors of serine proteases of the chymotrypsin family, although some have evolved to inhibit other types of serine proteases, and a few are also able to inhibit cysteine protease (Schick et al., 1998, McGowan et al., 2006, Vercammen et al., 2006, Ong et al., 2007, Roberts and Hejgaard, 2008). Furthermore, some serpins have the ability to form complexes with very divergent proteases (Huntington, 2006). Serpins are involved in a number of fundamental biological processes, and a role in the protection of storage tissue against insects and pathogens has been proposed for plant serpins (Dahl et al., 1996; Rasmussen et al., 1996).
1 Proteinase inhibitors in plants
Plants have developed defense systems to combat various pathogens throughout their life cycle, from the seed stage until senescence, and it is particularly important to keep embryo free from infection. There are several embryonic defense mechanisms detected, including the production of plant lectins and pathogen-related proteins, in response to pathogen or insect attack (Ye et al., 2001; Guiderdoni et al., 2002). Serine proteinase inhibitors are expressed in developing seeds and are thought to play an important role in inhibiting trypsin and chymotrypsin of external origin. Two major serine class of proteinase inhibitor shave been studied extensively in plants: Kunitz inhibitors and Bowman-Birk inhibitors (Ryan, 1990). Proteinase inhibitors of high molecular weight with low cystein content are termed as Kunitz type (Odani and Ikeneka, 1973). Bowman-Birk inhibitors (BBIs) are cystein rich proteins of about 8 kD to 16 kD with disulfide bonds. Serine proteinase inhibitors are universal and most studied class of proteinase inhibitors in plant kingdom (Haq et al., 2004, Mello et al., 2002). Although they are present in lower concentration in vegetative tissues, are primarily localized to storage tissues such as seeds and tubers rich in storage proteins.
Plant cystatins or phytocystatins are the second most studied class of inhibitors from plants, viz, cowpea, potato, cabbage, ragweed, carrot, papaya, apple fruit, avocado, chestnut, and Job’s tears. Seed cystatins have been reported from wide range of crops including sunflower, rice, wheat, maize, soybean, and sugarcane (Kuroda et al., 2001; Yozaura et al., 2002; Connors et al., 2002). Squash inhibitor, member of highly potent canonical serine proteinase inhibitors with typical knottin fold, was isolated and characterized (Chiche et al., 2004).
2 Families of proteinase inhibitors and their distribution
A comprehensive system of classification has been proposed for facilitating the exchange, storage and retrieval of information about this group of proteins (Rawlings et al., 2004). On the basis of three-dimensional structures, 31 families are assigned to 26 clans. The term “Clan” is to designate a single evolutionary line of inhibitors defined by single type of protein fold. Leo et al (2002) developed PLANT –PIs database to facilitate retrieval of information on plant protease inhibitors and related genes (Table 1). Christeller and Laing (2005) identified eight families of serine proteinase inhibitors (Table 2), which matched previously identified eight families in MEROPS.
Table 1 Plant proteinase inhibitor family in PLANT-Pis |
| Table 2 The building route of the NILs for restorer genes |
Soybean trypsin inhibitor was the first PI to be isolated and characterized. Since then many PIs have been characterized that are widely distributed throughout the plant kingdom (Konarev et al., 2004). Most of the plant PIs characterized are from Gramineae, Poaceae, Leguminosae, Fabaceae, and Solanaceae families (Brzin and Kidric, 1995). PIs are usually found in storage organs, such as seeds and tubes. However, their occurrence in aerial part of plants,as a consequence of several stimuli, has also been documented (De Leo et al., 2002). PIs accumulate to about 1 to 10% of the total soluble proteins of storage tissues. Number of PIs has been reported from non-storage tissues, such as leaves, flowers and roots (Brzin and Kidric, 1995; Xu et al., 2001; Sin and Chye, 2004). Trypsin inhibitor in mung bean is found localized to cytosol of cotyledonary cells (Chrispeels and Baumgartner, 1978). Soybean trypsin inhibitor (SBTI) was found localized in cotyledonary cell walls and embryonic cells, and to lesser extent in protein bodies, cytoplasm and nuclei. Soybean Bowman- Brik inhibitor (SBBI) was found to occur in protein bodies, nuclei, and cytoplasm (Horisberger and Tacchini-Vonlanthen, 1983). In tomatoes, serine proteinase inhibitors I and II selectively accumulated in endosperm and secretory cells of root cap (Narwaez-Vasquez et al., 1993). Xu et al (2004) reported the expression of phloem specific PIN2 protein in S. americanum stems, roots, and leaves.
3 Properties and regulation of plant PIs
Plants PIs are typical polypeptides composed of L-amino acids linked through peptide bonds, widespread in both monocots and dicot species (Ryan, 1990). Although the molecular size of PIs varies from 4 to 85 kD, majority of them are in the range of 8 kD to 20 kD (Hung et al., 2003). Plant PIs usually have high cysteine residues that form disulfide bridges contributing significantly to the stability of the inhibitors. Bowman-Birk type of Trypsin inhibitor from Brassica campestris seed (BCTI) of molecular size 8 kD, was found to be thermo stable, which is apparently related to the presence of the disulfide bridge (Hung et al., 2003). Plant PIs are low in methionione, histidine and tryptophan but are often rich in aspartic acid, glutamic acid, serine, arginine and lysine. Glycosylated plant PIs similar to mammalian glycoprotein proteinase inhibitors have not been reported so far.
Systemic signals associated with translocation of wound response include systemin, abscisic acid (ABA), hydraulic (variation potentials) and electrical signals (Malone and Alarcon, 1995). In tomato, it has been shown that protease inhibitor initiation factor (PIIF) is wound inducible, triggers the cascade of events leading to the synthesis of serine proteinase inhibitor in the whole plant (Bryant et al., 1976; Miège et al., 1976). This suggests the existence of signal that moves from injured tissue to all parts of the plant leading to systematic induction of PI gene expression. Systemin regulates the activation of over 20 defensive genes in tomato, in response to herbivore and pathogen attack, by activating lipid based signal transduction pathway- linolenic acid released from plant membranes is converted into signaling molecule, jasmonic acid (JA). Systemin, cell surface receptor of molecular size~160 kD, regulates intercellular cascade by (1) depolarization of the plasma membrane and (2) opening of ion channels, thus increasing the intracellular Ca2+, which activates MAPK and phospholipase A. These rapid changes play vital role in intra cellular release of membrane linolenic acid and its conversion to JA, potent transcription activator of defense gene (Koiwa et al., 1997; Ryan, 2000). Plant infected by pathogen trigger two possible signal pathways as its defense strategy- (1) recognition of penetration and colonization of pathogen through wound and (2) direct molecular recognition of the pathogen (Figure 1; Figure 2) (Cordero et al., 1994). Salicylic Acid (SA) and its methyl ester (MeSA) induce systemic acquired resistance in plants against pathogenic microorganisms (Hunt et al., 1996). Several jasmonic acid-dependent and independent wound signal transduction pathways have been identified and characterized. Components of these signaling pathways are similar to other signaling cascades that include reversible protein phosphorylation cascade, calcium/calmodulin- regulated events and production of reactive oxygen species (León et al., 2001). Stintzi et al (2001) demonstrated that in absence of JA, 1, 2-oxo- phytodienoic acid (OPDA), precursor of JA, elicits defense signal response. Induced rapid de novo synthesis of rice BBI was found in seedling/leaf in response to cut, exogenous application of jasmonic acid (JA), and protein phosphatase 2A (PP2A) inhibitors and completely inhibited by cycloheximide (Rakwal et al., 2001). Studies on herbivore-induced synthesis of glucosinolates, trypsin inhibitors, and resistance to herbivory in Brassica suggest that induced levels of trypsin inhibitors vary with genotypes (Cippolini et al., 2003). Changes in expression of jasmonic acid (JA)-and salicylic acid (SA)-dependent defense genes was observed in potato in response to potato and green peach aphids infestation (Martinez et al., 2003). Saedler and Baldwin (2004) demonstrated the potential of VIGS to manipulate and silence the expression of two jasmonate-induced genes that mediate the expression of proteinase inhibitor in Nicotiana attenuata roots and shoots. Hypothetical model for defense gene activation involving JA has been proposed which involves PI genes and jasmonate as critical signals in dicot’s defense responses (Doares et al., 1995; Sivasankar et al., 2000). Farmer et al (1992) hypothesized that lipases may be synthesized or activated in response to wounding and that linolenic acid released from membranes may initiate the intracellular transduction pathway leading to proteinase inhibitor synthesis. Free linolenic acid could be converted to jasmonic acid, which may be a key signaling component that may be very close to the trans-element(s) that regulate proteinase inhibitor gene transcription (Figure 1).
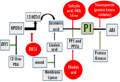
Figure 1 Regulation of proteinase inhibitor gene in plant
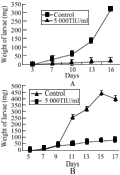
Figure 2 Larval growth curve of second (A) and third (B) instar Helicoverpa armigera larvae fed on artificial diet containing ChTI (5000 TIU/mL) and control on same diet without ChTI
Early events of signaling cascade involve changes in protein phosphorylation pattern, which eventually regulate various cellular processes in eukaryotes (Hunter, 1995), including plant defense responses (Conrath et al., 1997). Phosphorylation of proteins is a transient process, can be regulated by using protein phosphatase inhibitors- cantharidin and endothall (Li et al., 1993; MacKintosh et al., 1994; Millward et al., 1999). JA is found highly effective in inducing defense-related and cellular defense proteins, including PRs (Agrawal et al., 2000; Rakwal et al., 1999). It is initiated with the interactions of local or systemic signal molecules (Abscisic acid, systemin, oligogalacturonic acid, chitosan, electrical, and hydraulic signals) and putative plasma membrane receptors (β-glucan-elicitor-binding protein and systemin-binding protein). P-chloromercuribenzene sulfonic acid (PCMBS) has been shown to inhibit phloem systemin translocation, and attenuate systemic induction of protease inhibitor (PI) gene expression. Wound signal is transduced by an unidentified lipase that facilitates the release of linolenic acid from membrane lipids, a process stimulated by ABA. Volicitin, which originates from oral secretion of insects, also function like linolenic acid (Alborn, 1997). Def1 regulates the conversion of 13(S)- hydroperoxylinolenic acid (HPOTrE) to 12-oxo- phytodienoic acid (12-oxo-PDA). Def1 tomato mutant sensitive to insect attack is found defective in octadecanoid pathway. Diethyldi- thiocarbamic acid (DIECA) reduces the conversion of HPOTrE to 13-hydroxylinolenic acid (HOTrE) and decreases the synthesis of jasmonic acid (Howe, 1996). Inhibitors of ethylene action (norbornardiene (NBD) and silver) and salicylic acid prevent jasmonic acid-stimulated induction of PI expression (Borella, 1996; O’Donnell, 1996). Okadaic acid, inhibitor of protein phosphatase1 and 2A, inhibits jasmonate-induced PI expression; suggest the involvement of these phosphatases downstream regulatory pathway of jasmonate. The protein kinase inhibitor, staurosporine, inhibits PI gene expression induced by ABA, specifically, but not jasmonate (Dammann, 1997) (Figure 1).
4 Role of proteinase inhibitors in plants
Plant PIs are known to play important role in plant’s defense against insect-pest and pathogens as well as regulation of endogenous proteinases (Mosolov et al., 2001; Birk, 2003; Shewry, 2003). They are of interest in plant biology as source of (1) resistance against pests and pathogens, (2) drugs with antiviral properties and (3) markers for studying plant diversity and evolution (Konarev et al., 2002; Lawrence and Koundal, 2002; Korsinczky et al., 2004).
4.1 In insect pest control
Serine proteinases have been identified in gut extracts of many lepidopteran insects (Houseman et al., 1989) and many of these enzymes are inhibited by proteinase inhibitors. The pH of lepidopteran guts are alkaline, ranging from 9.0-11.0 (Applebaum, 1985) wherein serine- and metallo-proteinases are most active. Hence, serine proteinase inhibitors show anti-nutritional effects against several lepidopteran insects (Shulke and Murdock, 1983; Applebaum, 1985). Nandeesha and Prasad (2001) have reported partially purified subabul trypsin inhibitor (STI) from Leucaena leucacephala with molecular weight of 15 kD that shows high-level thermo tolerance and pH stability. Bioassay reveals that STI is a strong inhibitor of H. armigera, extending larval growth period by 12 days and induces mortality by 40% at 20,000 TIU/mL concentrations. Dimorphandra mollis seed trypsin inhibitor (DMTI-â…¡) showed 67% mortality among bruchid, (Callosobruchus maculates) fed at 1% level in artificial diet (Macedo et al., 2002). Giri et al (2003) reported at least 14 trypsin inhibitors from Psophocarpus tetragonolobus seeds. WBTI-1 (28 kD) was identified as a potent inhibitor of HGP activity (94%). WBTI-2 (24 kD) and WBTI-4 (20 kD) inhibited HGP activity up to 85%. WBTI-3-5-7 showed limited inhibition of HGP as compared with trypsin. Barley (Hordeum vulgare L.) malt contains all the four classes of endoproteinases as well as low molecular weight peptides that inhibit many of these endoproteinases. These are chloroform/ methanol soluble peptides, possibly play a significant role in controlling the activity of barley proteinases during germination, and protecting seed and young plant from pathogens/pests (Jones and Fontanini, 2003). Cotton boll weevil, Anthonomus grandis, feeds on fruits and buds causing severe crop loss. Trypsin/ chymotrypsin inhibitor (BTCI) purified from Vigna unguiculata seeds showed low inhibitory activity against trypsin-like proteinases of adult weevils. The bioassay results strongly suggest that BTCI has potential to engineer crop plants for resistance to the cotton boll weevil (Franco et al., 2003). Peltophorum dubium seed trypsin inhibitor (20 kD), thermo stable kunitz type inhibitor showed 56% mortality in Anagasta kuehniella at 1.6% level (Rodrigues et al., 2003). Chougule et al (2003) analyzed seeds of 53 pigeon pea cultivars and wild genotypes, resistant and susceptible to insect-pests and pathogens, for the presence of proteinase inhibitors against Helicoverpa armigera gut proteinases (HGPs). PIs from pigeonpea cultivars showed complete inhibition of trypsin and chymotrypsin, and moderately towards HGPs. PIs of wild relatives showed stronger inhibition with HGPs. Telang et al (2003) purified bitter gourd (Momordica charantia L.) seed proteinase inhibitors (BGPIs) that strongly inhibit HGPs. Electrophoretic analysis revealed the presence of two major proteins (BGPI-1 and-2) and two minor proteins (BGPI-3 and-4) having inhibitory activity against trypsin and HGPs. BGPIs inhibited proteolytic activity of larvae fed on different host plants, artificial diet with or without PIs supplementation and proteinases excreted in fecal matter from respective samples. BGPIs were found to retard growth and development of Helicoverpa armigera and Spodoptera litura. Reports indicate that BGPIs mediated inhibition of insect gut proteinases directly affect fertility and fecundity of H. armigera and S. litura. Patricia et al (2003) used bioassays to investigate the effect of Bowman Birk and kunitz -type soybean trypsin inhibitor on growth pattern of Diatraea saccharalis moth using two diets- Diet 1 was less nutritious, with low protein content; and reduced minerals and essential aminoacids (cysteine, lysine and methionine) content while Diet 2 was richer and more complete. Food intake and utilization; larval development and mortality were monitored. When PI was supplemented, larval development was significantly altered in larvae fed with diet1, with reduced trypsin-like activity of midgut enzymes. Diet 2 fed larvae also showed reduced level of trypsin-like activity but it was less marked than diet 1. Similar feeding experiment was done with subabul high and low molecular weight trypsin inhibitor (HSTI, LSTI) using artificial diet, chickpea seeds and leaves. Larvae fed with artificial diet showed reduction in larval weight up to 21% (HSTI) and 43% (LSTI). However, larvae fed on seeds showed significant reduction in weight, 52.4% (HSTI) and 60.3% (LSTI), suggesting the diet also play vital role on the effectiveness of the inhibitors on larval growth and development (Bhavani et al., 2007). Franco et al (2004) suggested that SKTI can be an effective in developing transgenic plants against the cotton boll weevil, Anthonomus grandis. Cotton boll weevil gut digestive system contains serine proteinases. In vitro assay showed that SKTI inhibit these enzymes. Neonate larvae reared on an artificial diet containing SKTI showed reduction in larval weight of up to 64% and caused mortality and severe deformities of larvae, pupae and adult insects. Oryza sativa chymotrypsin inhibitor (OCPI1) transgenic positive plants showed higher grain yield and seed setting rate than the wild type and control under the severe drought stress conditions, whereas the potential yield of transgenic plants under normal growth conditions was not affected. Chymotrypsin- inhibitor activity assay from positive transgenic plants showed stronger inhibition. The decrease of total proteins in transgenic plants is less than the wild type under drought stress (Huang et al., 2007). The defensive role of PIs is based on their inhibitory activities towards proteolytic enzymes of insect gut and phytopathogens resulting either in a critical shortage of essential amino acids (Hilder et al., 1993; Jongsma and Bolter, 1997) or interfering with metabolic processes, such as the proteolytic activation of enzymes, molting of insects, or replication of viruses (Gutierrez-Campos et al., 1999). Direct evidence for the involvement of PIs in the plant defense system has come from studies on transgenic plants. Transgenic plants expressing PIs have been produced in the last two decades and tested for enhanced defense capacities, particularly against insect-pests (De Leo et al., 2002). Expression of cowpea trypsin inhibitor (CpTI) in transgenic tobacco was shown for the first time to confer resistance to feeding by tobacco budworm Heliothis virescens (
. PDF(396KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Chumki Bhattacharjee
. Doddananjappa Theertha Prasad
. Nagenahalli Huchappa Manjunat
. Debarshi Sanyal
. Sajad Majeed Zargar
Related articles
. Serine proteinase inhibitor
. Trypsin inhibitor
. Insect pest
. Cloning
Tools
. Email to a friend
. Post a comment


