2. UPASI Tea Research Foundation, Tea Research Institute, Nirar Dam BPO, Valparai-642 127, Tamil Nadu, India
 Author
Author  Correspondence author
Correspondence author
Molecular Soil Biology, 2013, Vol. 4, No. 1 doi: 10.5376/msb.2013.04.0001
Received: 20 Dec., 2012 Accepted: 09 Jan., 2013 Published: 17 Feb., 2013
Sarkar et al., 2012, Hexavalent Chromium (Cr (VI)) Removal by Live Mycelium of a Trichoderma harzianum Strain, Molecular Soil Biology, Vol.4, No.1 1-6 (doi: 10.5376/msb.2013.04.0001)
Response of Trichoderma harzianum strain to different chromium concentrations was investigated by poison food technique. It was noticed that, the mycelial growth was inhibited up to 94 % at 40 mg/L concentration followed by 30 mg/L (91%).The chromium (VI) biosorption ability of Trichoderma harzianum was tested in vitro. The organism was inoculated on Czapek Dox broth medium containing 30 mg/L of Cr (VI) salt. The metal residues were analyzed at different day's interval (4, 5, 6 and 7 days). The effect of different pH and temperature on metal removal was also investigated. Results indicated that, at 7th day the metal removal reached the maximum level (90.2%). Further incubation did not increase the metal uptake. A pH range of 4~5 and temperature of 30℃ was optimum for Cr (VI) removal by T. harzianum in the present study.
Introduction
One of the most common and abundant heavy metal in the earth crust is chromium (IARC. 1973). This metal is also widely used in several industrial processes like metal cleaning, textile, dyes etc. (Sen and Ghosh Dastidar, 2007; Morales-Barrera and Cristiani-Urbina, 2008). Cr can occur in various oxidation states (-2 to +6), but commonly found in oxidation states of +3 and +6. The oxidation states have significant consequences for toxicity, bioavailability and enrichment by microbial biomass (Bartlett, 1991). The trivalent Cr compounds are less noxious, less portable and available for biological uptake, while hexavalent Cr are more toxic due to its greater solubility in water, rapid permeability and subsequent interaction with cell components (Sultan and Hasnain, 2005). Cr (VI) also represents health hazards to animals and humans since they are reported to be toxic, mutagenic, carcinogenic and teratogenic (McLean and Beveridge, 2001; JECFA, 2005). Existing methods presently employed to eliminate heavy metals are many like precipitation, ion-exchange, electrodeposition, reverse osmosis etc (Rodriguez et al., 2006; Alluri et al., 2007). These techniques for metal removal are not often suitable or not very cost effective (Al-Saraj et al., 1999; Vieira and Volesky, 2000) and often generate other wastes that require further treatments (Rostami and Joodaki, 2002). Due to these complications, an alternative way of metal removal is of great importance.
The ability of microorganisms to take up metals has been confirmed for some time (Hussain et al., 2004; Preetha and Viruthagiri, 2005). The possible use of microorganisms in treatment of hazardous materials and metals from aqueous environment by biosorption is considered as a favoured means (Ma et al., 2004; Viraraghavan and Yan, 2003). Fungi, with other microbial groups can accumulate metals from external environments by means of various ways like physico-chemical and biological mechanisms (Cabuk et al., 2005). This technique of metal removal has attracted increased attention in recent years which offers several advantages over conventional methods (Popuri et al., 2007). In several previous studies, metal removal abilities of various fungi have been investigated like Talaromyces helicus (Romero et al. 2006), Rhizopus arrhizus (Subudhi and Kar, 2008), Polyporus squamosus (Wuyep et al., 2007), Trichoderma reesei (Kim et al., 2003), Cunninghamella echinulata (El-Sayed and El-Morsy, 2004), Aspergillus niger (Awofolu et al., 2006), Penicillium chrysogenum (Niu and Volesky, 1999). However there is no sufficient data available on the metal removal capacity of Trichoderma harzianum. So in the present study an attempt was made to study the Cr (VI) removal ability of T. harzianum in vitro. The effect of different physical parameters like pH and temperature on metal removal was also investigated.
1 Material and Methods
1.1 Organism and culture conditions
A strain of Trichoderma harzianum was obtained from center for advance studies in botany (CAS), University of Madras, and was routinely maintained on readymade potato dextrose agar (PDA) of HI- MEDIA make.
1.2 Reagents
Metal stock solution was prepared by dissolving potassium dichromate (K2Cr2O7) salt of SRL AR grade in distilled water (DW).
1.3 Sensitivity/Tolerance of T. harzianum strain to Cr (VI)
In vitro sensitivity/tolerance of T. harzianum strain to different concentrations of Cr (VI) was determined by poisoned food technique (Dhingra and Sinclair, 1985). Appropriate quantity of the Cr (VI) stock solution was added to molten Czapek Dox agar (CDA) medium to get the required concentration (5, 10, 20, 30 and 50 mg/L) and poured in to sterilized petri plates (9 cm) after gentle shaking. Metal un-amended medium served as the control. The plates were inoculated by placing 5 mm discs of 4 days old culture of T. harzianum strain and incubated at room temperature (25 ± 2)℃. Inhibition of radial growth was measured based on colony diameter, by using the formula stated by Sundar et al (1995).
Percent Inhibition (PI) = [(X-Y/X) x 100]
Where,
X = Radial growth (mm) of control plates
Y = Radial growth (mm) of treated plates
1.4 Biomass preparation
For preparation of the fungal biomass, T. harzianum strain was inoculated in CDA plates and incubated at room temperature. After 5 days, a small portion (0.5 mm) of the fungus mycelium was cut and transferred into 200 mL Czapek Dox broth (CDB) broth in a 500 mL Erlenmeyer flask and incubated at 27℃. After the incubation period, the pellets thus formed were harvested from the medium, washed thrice with sterilized distilled water and stored at 4℃ until further studies.
1.5 Biosorption of Cr (VI)
The biosorption experiment was conducted in Erlenmeyer flask (500 mL) containing 200 mL of Czapek Dox broth (CDB) and known concentration of Cr (VI) solution in triplicates. T. harzianum pellets were inoculated in to the flask and incubated at different day’s intervals (4, 5, 6 and 7 days). Measurement of Cr (VI) residue in the growth medium was conducted with Perkin- Elmer A-Analyst, AA800 (Perkin-Elmer Corporation, Shelton, USA) atomic absorption spectrophotometer.
The adsorption isotherm was calculated by Freundlich and Langmuir isotherm pattern using the following formulas:
Freundlich isotherm
log (x/m) =1/n log C+ log K
Where, x/m is the amount of metals adsorbed (mg of Cr kg-1), C is the equilibrium concentration in soil solution and n and K is the constants of adsorption isotherms. Values of log K represent the amount of metals adsorbed at unit concentration and 1/n represents the concentration gradient.
Langmuir adsorption isotherms
C/x = 1/ (K n) + C/n
Where C= equilibrium concentration of Cr, x is the amount of metals adsorbed, K is the constant related to binding energy and n is the metals adsorption maxima. From a linear plot of C/x verses C, adsorption maxima was calculated, as the inverse of the slope and constant related to bonding energy was determined as slope (or) intercept.
1.6 Effects of pH and temperature on Cr (VI) removal
To study the effect of different pH and temperature on Cr (VI) removal, CDB was amended with known concentration of Cr (VI) solution and adjusted to different pH (2,3,4,5 and 6) by using 0.1N HCl and 0.1N NaOH. T. harzianum strain was inoculated in the medium and incubated at different temperature (10℃~40℃). Measurement of Cr (VI) content in the growth medium was measured as mentioned above.
2 Results and Discussion
Influence of Cr (VI) on mycelial development of T. harzianum strain is presented on Table 1. A progressive increase in percent inhibition was observed with increase in initial concentration of Cr (VI). Among the different concentrations of Cr (VI) tested, 40 mg/L was the most lethal, where the growth inhibition was 94%, followed by 30 mg/L (91%). Metal removal capacity of T. harzianum strain is given in figure 1. It was noticed that the metal removal to a certain extent was time dependent process. A progressive increase in Cr (VI) absorption was noticed with an increase in incubation days. At 7th day incubation, the metal uptake was maximum (90.2%). Further incubation did not change the capacity of metal removal significantly. This may be due to the saturation of the fungal mycelia in metal uptake. Previous studies reported that metal-ion uptake of biosorbent increases as long as the biosorbent is not saturated (Fourest and Roux, 1992).
.png) Table 1 Sensitivity/Tolerance of T. harzianum strain to Cr (VI) |
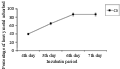 Figure 1 Biosorption of Cr (VI) by T. harzianum at different days |
The adsorption of hexavalent chromium followed the typical Freundlich and Langmuir isotherm pattern (Table 2). In the case of Freundlich adsorption isotherms the plot made between log x / m verses log C was found to be straight line for Cr (VI). The Freundlich coefficient ‘K’ is regarded as the hypothetical index of heavy metal sorbed from a solution having unit equilibrium concentration (Ghosal et al., 2003). Hypothetical index was found to be 1.753 in the present study. Considering the fit value (96%), it is suggested that the adsorption of heavy metals by T. harzianum can use Freundlich adsorption isotherm to evaluate the adsorption characteristics. In case of Langmuir adsorption isotherm, the plot made between C/ (x/m) and C was found to be straight line. In general, Langmuir adsorption isotherms were having fit value 94% in the present study. Therefore this isotherm can also be used to predict the metal adsorption characteristics of T. harzianum. In case of adsorption maxima and binding energy, the value was higher (570.16 and 0.405 respectively) which indicated the metal adsorption and retaining capacity of T. harzianum was superior in case of Cr (VI). The mycelium after the study were re suspended in water to observe any leakage of the adsorbed metals to the surroundings. No residues were detected in the water up to 10 days, which indicated the binding tendencies of the T. harzianum mycelium towards the tested chromium metal (data not shown).
.png) Table 2 Constants and correlation coefficients for the Langmuir and Freundlich isotherms |
The effect of different pH on metal removal is presented in Figure 2. At lowest pH tested in the present study (pH 2), no metal removal was observed. A gradual increase in pH demonstrated increased metal removal capacity by T. harzianum. Similar kind of results was also noticed by other researchers (Tian– Wei et al., 2004; Wuyep et al., 2007). The less bioaccumulation capacity at lower pH is reported due to the competition of hydrogen ion with metal ion on the sorption sites (Congeevaram et al., 2007). It has been reported that, sorption of heavy metals by the fungi is strongly pH dependent and biosorption rate increases with increase in pH (Lovely, 1995). Metal removal was noticed between pH 3 to 5 in the present study with the maximum metal removal in pH 4. Several authors previously described an optimal pH around 4 is an ideal condition for metal removal (Tobin et al., 1984; Tsezos and Volesky, 1981). At pH value above 7, metals exist as hydroxide colloids and precipitate at alkaline pH due to osmotic changes and hydrolyzing effect (Nasseri et al., 2002), thus resulting reduced uptake rate.
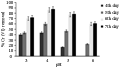 Figure 2 Biosorption of Cr (VI) by T. harzianum at different pH |
Temperature plays a significant role in the biosorption of metal ions. An increase in temperature showed increased metal removal to certain point. The optimum temperature for maximum Cr (VI) removal was noticed at 30℃ in the present study (Figure 3). At this temperature, the metal removal was in the range of 90%. Srivastava and Thakur (2006) suggested that 30℃ was the ideal condition for bioaccumulation of chromium in case of Aspergillus sp. The metal uptake was decreased beyond this temperature. At 40℃ the metal removal was reduced to 20% whereas, no absorption was noticed at 50℃. Similar report was suggested by Bai and Abraham (2001), where a decline in Cr (VI) biosorption was noticed at 50℃. Though temperature plays an important role for the growth of organisms, at elevated level, it damages the organisms by denaturing enzymes, transport carriers, integrity of cell membrane (Prescott et al., 2002), and also hinder compart- mentalization of metal ions leading to reduced metal uptake (Fartal et al., 2007). The present in vitro studies indicated that Trichoderma harzianum strain was able to uptake substantial amount of Cr (VI) from aqueous medium. A temperature of 30℃ and pH range of 3 to 5 was perfect for the maximum metal removal in the present study.
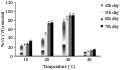 Figure 3 Biosorption of Cr (VI) by T. harzianum at different temperature |
Author’s Contributions
SS, AS and RP collected and systematized data, SS performed the statistical analyses and the manuscript. All the authors have read and approved the final manuscript.
Acknowledgements
The authors are thankful to Dr. N. Muraleedharan, Adviser and Dr. P. Mohan Kumar, Director of UPASI Tea Research Institute for their constant encouragement and support.
References
Alluri H.K., Ronda S.R., Settalluri V.S., Bondili J.S., Suryanarayana V., and Venkateshwar P., 2007, Biosorption: An eco-friendly alternative for heavy metal removal, Afr. J.Biotechnol, 6: 2924-2931
Al-Saraj M., Abdel- Latif M.S., I. El-Nahal I., and Baraka R., 1999, Bioaccumulation of some hazardous metals by sol- gel entrapped microorganisms, J. Non- Crysst Solids, 248: 137-140
Awofolu O.R., Okonkwo J.O., Merwe R.R.D., Badenhorst J., and Jordaan E., 2006, A new approach to chemical modification protocols of Aspergillus niger and sorption of lead ion by fungal species, Electron, J. Biotechnol, 9: 340-348
Bai R.T., and Abraham E., 2001, Biosorption of Cr (VI) from aqueous solution by Rhizopus nigricans, Bioresour Technol, 79: 73-81 http://dx.doi.org/10.1016/S0960-8524(00)00107-3
Bartlett, R J., 1991, Chromium cyclings in soil and water: links, gaps and methods, Environ Health Persp, 92: 17-24 http://dx.doi.org/10.1289/ehp.919217 PMid:1935847 PMCid:1519379
Cabuk A., Ä°lhan S., Filik C., and Caliskan F., 2005, Pb2+ biosorption by pretreated fungal biomass, Turk. J. Biol., 29: 23-28
Congeevaram S., Dhanarani S., Park J., Dexilin M., and Thamaraselvi K., 2007, Biosorption of chromium and nickel by heavy metalresistant fungal and bacterial isolates, J. Hazard. Mat., 146: 270-277 http://dx.doi.org/10.1016/j.jhazmat.2006.12.017 PMid:17218056
Dhingra O.D., and Sinclair J.B., 1985, Basic Plant Pathology Method, (2nd Eds), pp.434
El-Sayed M., and El-Morsy, 2004, Cunninghamella echinulata a new biosorbent of metals ions from polluted water in Egypt, Mycologia, 96: 1183-1189 http://dx.doi.org/10.2307/3762133
Faryal R., Yusuf M., Munir M., Tahir F., and Hameed A., 2007, Enhancement of Cr 6+ removal by Aspergillus niger RH 19 using a biofermenter, Pak J. Bot., 39: 1873-1881
Fourest E., and Roux J.C., 1992, Heavy metal biosorption by fungal mycelial by-products: mechanisms and influence of pH, Appl. Microbiol. Biotechnol, 3: 399-403 http://dx.doi.org/10.1007/BF00211001
Hussein H., Ibrahim S.F., Kandeel K., and Moawad H., 2004, Biosorption of heavy metals from waste water using Pseudomonas sp., Electron. J. Biotechnol, 7: 38-42
http://dx.doi.org/10.2225/vol7-issue1-fulltext-2
IARC., 1973, Review of carcinogenicity studies of chromium and chromium compounds, IARC monographs, 2: 100-125
JECFA, 2005, (Joint FAO/WHO Expert Committee on Food Additives), Safety Evaluation of Certain Food Additives and Contaminants WHO Food Additives Series, 44, World Health Organisation, Geneva
Kim S.K., Chun B.P., Yoon M.K., and Hyun S.Y., 2003, Biosorption of cadmium and copper by Trichoderma reesei RUT C30, J. Ind. Eng. Chem., 9: 403-406
Liu N., Luo S., Yang Y., Zhang T., Jin J., and Liao J., 2002, Biosorption of americium-241 by Saccharomyces cerevisiae, J. Radioanal. Nucl. Chem., 252: 187-191
http://dx.doi.org/10.1023/A:1015276813386
Ma B.Y., Li W., Jing G.H., and Shi Y., 2004, Dissimilatory reduction of Fe III (EDTA) with microorganisms in the system of nitric oxide removal from the flue gas by metal chelate adsorption, J. Environ Sci, 16: 428-430
Mclean J., and Beveridge T.J., 2001, Chromate reduction by a pseudomonad isolated from a site contaminated with chromate copper arsenate, Appl. Env. Microbiol., 67: 1076-1084
http://dx.doi.org/10.1128/AEM.67.3.1076-1084.2001
PMid:11229894 PMCid:92697
Morales-Barrera L., and Cristiani-Urbina E., 2008, Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent, Water Air Soil Pollut, 187: 327- 336
http://dx.doi.org/10.1007/s11270-007-9520-z
Nasseri S, Mazaheri A.M., Noori S.M., Rostami K.H., Shariat M., and Nadafi K., 2002, Chromium removal from tanning effluent using biomass of Aspergillus oryzae, Pak. J. Biol. Sci., 5: 1056-1059
http://dx.doi.org/10.3923/pjbs.2002.1056.1059
Niu H., and Volesky B., 1999, Characteristics of gold biosorption from cyanide solution, J. Chem. Tech. Biotechnol, 74: 778-784
http://dx.doi.org/10.1002/(SICI)1097-4660(199908)74:8<778::AID-JCTB99>3.0.CO;2-Q
Preetha B., and Viruthagiri T., 2005, Biosorption of zinc (II) by Rhizopus arrhizus: equilibrium and kinetic modelling, Afr. J. Biotechnol., 4: 506-508
Popuri S.R., Kalyani S., Kachireddy S.R., and Krishnaiah A., 2007, Biosorption of hexavalent chromium from aqueous solution by using prawn pond algae (Sphaeroplea), Indian J. Chem., 46A: 284-289
Prescott L.M., Harley J.P., and Klein D.A., 2002, Microbiology, (5th Eds): The McGraw-Hill Companies, Inc. North America, pp.1026
Rodriguez C.E., Quesada A., and Rodriguez E., 2006, Nickel biosorption by Acinetobacter baumannii and Pseudomonas aeruginosa isolated from industrial wastewater, Braz. J. Microbiol., 37: 465-467
http://dx.doi.org/10.1590/S1517-83822006000400012
Romero M.C., Reinoso E.H., Urrutia M.I., and Kiernan A.M., 2006, Biosorption of heavy metals by Talaromyces helicus: a trained fungus for copper and biphenyl detoxification, Electron. J. Biotechnol, 9: 221-226
http://dx.doi.org/10.2225/vol9-issue3-fulltext-11
Rostami K.H., and Joodaki M.R., 2002, Some studies of cadmium adsorption using Aspergillus niger, Penicillium austurianum, employing an airlift fermenter, Chem. Engi., 89: 239-252
http://dx.doi.org/10.1016/S1385-8947(02)00131-6
Sen M., and Ghosh Dastidar M., 2007, Biosorption of Cr (VI) by resting cells of Aspergillus sp., Iran J. Environ. Health. Sci. Eng., 4: 9-12
Srivastava S., and Thakur I.S., 2006, Isolation and process parameter optimization of Aspergillus sp. for removal of chromium from tannery effluent, Bioresour Technol., 97: 1167-1173
http://dx.doi.org/10.1016/j.biortech.2005.05.012
PMid:16023341
Subudhi, E., and Kar R.N., 2007, Biosorption of Rhizopus arrhizus biomass to accumulate zinc from aqueous solution: In: Mineral biotechnology, edited by Mishra et al., published by Director, Regional Research Lab., Bhubaneswar, pp.147-155
Sultan S., and Hasnain S., 2005, Chromate reduction capability of a gram positive bacterium isolated from effluent of dying industry, Bull. Environ. Contam. Toxicol, 75: 699-706
http://dx.doi.org/10.1007/s00128-005-0808-7
PMid:16400550
Sundar A.R., Das N.D., and Krishnaveni D., 1995, In-vitro antagonism of Trichoderma sp. against two fungal pathogens of Castor, Indian J. Plant Protec., 23: 152-155
Tian-Wei T., Hu B., and Haijia S., 2004, Adsorption of Ni2+ on amine-modified mycelium of Penicillium chrysogenum, Enzyme Microb.Technol., 89: 207-211
Tobin J. M., Cooper D.G., and Neufeld R.J., 1984, Uptake of metal ions by Rhizopus arrhizus, Appl. Environ. Microbiol., 47: 821-824
PMid:16346521 PMCid:239770
Tsezos M., and Volesky B., 1981, Biosorption of uranium and thorium, Biotechnol Bioeng, 23: 583-604
http://dx.doi.org/10.1002/bit.260230309
Vieira R., and Volesky B., 2003, Biosorption: a solution to pollution? Int. Microbiol., 3: 17-24
Viraraghavan T., and Yan G., 2003, Heavy metal removal from aqueous solution by fungus Mucor rouxii, Water Res., 37: 4486-4496
http://dx.doi.org/10.1016/S0043-1354(03)00409-3
Wuyep P.A., Chuma A.G., Awodi S., and Nok A.J., 2007, Biosorption of Cr, Mn, Fe, Ni, Cu and Pb metals from petroleum refinery effluent by calcium alginate immobilized mycelia of Polyporus squamosus, Scientific Research and Essay, 2: 217-221
. PDF(185KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Soumik Sarkar
. Annamalai Satheshkumar
. Robert Premkumar
Related articles
. Chromium
. Trichoderma harzianum
. Biosorption
. Ph
. Temperature
Tools
. Email to a friend
. Post a comment


