Author
Author  Correspondence author
Correspondence author
Molecular Soil Biology, 2015, Vol. 6, No. 3 doi: 10.5376/msb.2015.06.0003
Received: 19 May, 2015 Accepted: 01 Jun., 2015 Published: 07 Jun., 2015
The present study used the seeds and newborn seedings of Salix sungkianica as material, examining the influence of NaCl and NaHCO3 solutions of different concentrations on the seed germination and anatomical structure of the roots and lamina of the seedings. The results indicate such facts: (1) The presents of NaCl and NaHCO3 have a depressant effect on the seed germination, while a light concentration of NaHCO3 could accelerate the sprout. (2) S. sungkianica seedings show strong endurance under the stress of 50 mmon/L NaCl and 10 mmon/L NaHCO3 solutions, the seedings growth are better in 10 mmon/L NaHCO3. (3) Under the stress of such solutions of high concentration, certain degrees of endurance also present in the roots and lamina, with the anatomical results like the thicken of root endodermis, the enlargement of vascular bundle in diameter, the vanish of aerenchyma tissures and the closure of lamina stoma.
Transporters play one of the most fundamental roles in life, namely, selective import or removal of molecules across biological membranes. In Arabidopsis approximately 3% of the genome appears to code for transporters (The Arabidopsis Genome Initiative, 2000). A vast majority of these transporters are secondary transporters, which are energized by the proton gradient and membrane potential. The plant cation⁄H+ exchangers (CAXs) are part of the ensemble of transporters that coordinate the redistribution of various cations including calcium (Ca2+) in exchange for the protons generated by H+-pumps.
To date, there are over 200 full-length CAX cDNAs or predicted open reading frames (ORFs) in the GenBank database (Manohar et al., 2010). CAXs are one of the five closely related families of transporters that together form a large phylogenetic clade, termed the Ca2+⁄cation antiporter (CaCA) superfamily (Shigaki and Hirschi, 2006). These CAXs have been classified into three major categories based on phyogenetic analysis: Types Ⅰ (CAXs similar to A. thaliana CAX1), Ⅱ (CAXs similar to S. cerevisiae VNX1) and Ⅲ (CAXs similar to E. coli ChaA) (Shigaki et al., 2006).
The past several years has focused on the physiological roles, biochemistry and molecular biology of CAXs. CAXs are now recognised to be involved in a number of important aspects of plant growth and development (Conn et al., 2011) and may be excellent tools for nutritional enhancement of crops and mitigating pollutants in soils. We will summarise in this review the knowledge of subcellular localization of CAX proteins in plants.
1 Subcellular localization of CAX proteins in Arabidopsis thaliana
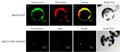
To further establish the subcellular localization of CAX1 in plants, microsomal membranes from wild-type and transgenic lines harboring the hemagglutinin (HA)-tagged truncated CAX1 fusion protein (HA-sCAX1) were fractionated. Centrifugation through a continuous Suc gradient was first used to compare the distribution of the epitope-tagged transporter in Arabidopsis (Figure 1) and the native full-length CAX1 (Figure 1) with that of markers for the vacuole, plasma membrane, and endoplasmic reticulum. As shown in Figure 1, when membrane fractions were assayed for CAX1 and HA-sCAX1 accumulation, proteins of 50 kD increased at 30% Suc (most abundant in fractions corresponding to 28% and 37% Suc, respectively). Both endogenous CAX1 and HA-sCAX1 accumulated in fractions enriched in vacuolar membranes, which overlap with that of a resident protein (V-PPase) from this membrane.CAX2 contains 11 putative transmembrane domains and has a predicted molecular mass of 39 kD (Hirschi et al., 1996). In yeast this protein appears to function at the tonoplast membrane (Hirschi et al., 1996). To identify the cellular localization of CAX2 in plants, researchers produced polyclonal antibodies against a peptide from the deduced amino acid sequence of the central nonmembranal loop. As shown in Figure 2, western-blot analysis of Arabidopsis membranes fractionated on Suc gradients show that CAX2 cofractionates with the vacuolar membrane marker tonoplast intrinsic protein, and not with plasma membrane or endoplasmic reticulum markers. Thus, CAX2 is predominately localized in the vacuolar membrane.
.png) Figure 1 Subcellular Localization of CAX1 in Arabidopsis. Immunoblot analysis of CAX1 in Arabidopsis. Equal amounts of protein (10 g) isolated from HA-sCAX1-expressing transgenic Arabidopsis, wild-type Arabidopsis, and plant membrane markers: the plant endoplasmic reticulum luminal protein (BiP), mung bean vacuolar pyrophosphatase (V-PPase). Wild-type Arabidopsis membranes were prepared and fractionated in the absence or presence of 5 mmol/L MgCl2, and then assayed for changes in CAX1 and BiP expression. Numbers above the gels indicate the Suc concentration of each fraction |
 Figure 2 Intracellular localization of CAX2 in wild-type Arabidopsis plants. Arabidopsis membranes were extracted and fractionated in a Suc gradient. The fractions (fraction 1=21%; fraction 7=38% [v/v] Suc) were subjected to western-blot analyses using the following antibodies: CAX2, affinity-purified antibodies against a peptide from CAX2 deduced amino acid sequence; VM, antibodies against a vacuolar membrane marker VM23, a homolog of tonoplast intrinsic protein from radish (Raphanus sativus), which is a species closely related to Arabidopsis (Maeshima, 1992); PM, antibodies against the Arabidopsis plasma membrane marker protein RD-28 (Yamaguchi et al., 1992); ER, antibodies against the endoplasmic reticulum yeast BiP protein that specifically recognize plant endoplasmic reticulum BiP (Shimoni et al., 1995) |
The localization of HA: CAX4 on the vacuolar membrane of plants was shown by the heterologous expression of the CAX4 fusion protein in a suspension of tobacco cells. The fusion protein under the control of the cauliflower mosaic virus 35S promoter was expressed in tobacco BY-2 cells. In the case of BY-2 cells, many vacuoles are generally observed in a single cell (Kost et al., 1998). As shown in Figure 3, A and B, confocal images of red fluorescent signals stained by Texas Red conjugated antibody revealed that HA: CAX4 is localized in the vacuolar membrane.
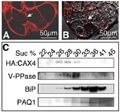 Figure 3 Expression of HA: CAX4 in tobacco BY-2 cells. A and B, Immunostaining of HA: CAX4 in BY-2 cell. A, HA: CAX4 was detected on vacuolar membrane by HA antibody and Texas Red conjugated secondary antibody (indicated by arrow). B, A superimposed image of red channel and transmitted light demonstrated only vacuolar membranes were stained (indicated by arrow). C, Immunoblotting of HA: CAX4 in tobacco BY-2 cells. Monoclonal antibodies against HA and a plant endoplasmic reticulum luminal protein (BiP), an indicator of endoplasmic reticulum, were used at dilutions of 1:1,000 and 1:1,500, respectively. Polyclonal antibodies against mung bean (Vigna radiata) vacuolar pyrophosphatase (V-PPase) and radish plasma membrane aquaporin (PAQ1) were used at 1:1,000 dilutions. The numbers indicate the concentration of the Suc fraction |
To confirm vacuolar membrane localization of the fusion protein, the crude membrane fraction of tobacco cells was subjected to Suc gradient centrifugation and immunoblotting with the anti-HA antibody. The distribution of the fusion protein (fractions from 26% to 33%) was paralleled by that of a vacuolar marker (mung bean vacuolar pyrophosphatase) and not that of an endoplasmic reticulum marker (plant endoplasmic reticulum luminal protein) and a plant plasma membrane marker (radish plasma membrane aquaporin; Figure 3C).
2 Subcellular localization of CAX protein in Vigna radiata
The localization of VCAX1p on the vacuolar membrane was confirmed by the heterologous expression of VCAX1p in a suspension of tobacco cells. In this experiment, the VCAX1 sequence was fused at the C-terminus of sGFP and introduced into tobacco BY-2 cells via Ag. tumefaciens to express the fusion protein under the control of the CAMV 35S promoter. In the case of BY-2 cells, many vacuoles were generally observed in a single cell. Confocal imaging of GFP fluorescence emitted from transformed cells revealed that sGFP-VCAX1p is localized in the vacuolar membrane and small vesicles (Figure 4). No green fluorescence was detected on the plasma membrane, nuclear envelopes or free cytosol.
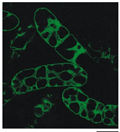 Figure 4 Localization of sGFP-VCAX1p fusion protein expressed in tobacco suspension cells. A fusion protein of sGFP and VCAX1p was expressed in tobacco BY-2 cells, and then sGFP green fluorescence of the cells was imaged under a laser scanning confocal microscope. Scale bar: 20 µm |
3 Subcellular localization of CAX protein in Glycine max
A green fluorescent protein (GFP)-tagged version of GmCAX1 was made into a plant expression vector and then transformed and expressed in Arabidopsis. The GFP fluorescence in the guard cells of mature leaf was the most intensive. Optical sectioning through the center of the cell verified that the fluorescent signal was localized to the periphery of stomata in guard cells (Figure 5D~F), whereas the GFP alone was localized in the entire guard cells (Figure 5A~C). The result suggests that the GmCAX1-GFP fusion protein may be localized on the plasma membrane. To further investigate the GmCAX1 localization, the GmCAX1- GFP fusion gene was also introduced into the onion epidermal cells by particle bombardment. Before plasmolysis, the green fluorescence was mainly localized in the peripheral region of the cell (Figure 5I, J). After plasmolysis, the green fluorescence in the GmCAX1-GFP fusion protein appeared to be localized on the plasma membrane (Figure 5K, L). The strong fluorescence aggregates in Figure 5K possibly indicate the shrinkage of the membranes at the two poles of the cell and around the nucleous. The control GFP protein was mainly present in the cytosol portion (Figure 5G, H).
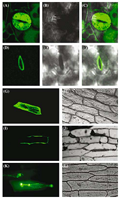 Figure 5 Subcellular localization of the GmCAX1-GFP fusion protein in plant cells. A~C, Localization of the control GFP protein in guard cells of transgenic Arabidopsis plants overexpressing the GFP gene. D~F, Localization of the GmCAX1-GFP fusion protein in guard cells of the transgenic plants overexpressing the GmCAX1-GFP fusion gene. G and H, Localization of the control GFP protein in an onion epidermal cell. I and J, Localization of the GmCAX1-GFP in the peripheral region of an onion epidermal cell before plasmolysis. K and L, Localization of the GmCAX1-GFP in the plasma membrane of an onion epidermal cell after plasmolysis. The strong fluorescence aggregates indicate the shrinkage of the membrane at the two poles and around the nucleous. The photographs were taken in the dark field for green fluorescence (parts A, D, G, I and K), in the bright light for the morphology of the cells (parts B, E, H, J and L), and in combination (parts C, F) |
4 Subcellular localization of CAX protein in Oryza sativa
Subcellular localization prediction (http://psort.nibb. ac.jp) showed that OsCAX3 is located on the plasma membrane of plant. The pBI221-GFP and pBI221- OsCAX3-GFP fusion vector were transformed into Arabidopsis mesophyll protoplast. Results showed, in the GFP control cells, fluorescence was distributed in cytoplasm and nucleus; while in the OsCAX3-GFP cells, fluorescence was distributed in plasma membrane (Figure 6). This shows that OsCAX3 is a plasma membrane Ca2+/H+ antiporter.
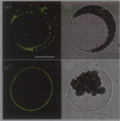 Figure 6 Transient expression and subcellular localization analysis of OsCAX3-GFP fusion protein in mesophyll cell protoplast of Arabidopsis (a) Confocal microscopy images of green fluorescence in protoplasts expressing GFP alone. (c) Optical sectioning microscopy image of green fluorescence in protoplasts expressing OsCAX3-GFP. (b), (d) the same cells of (a) and (c) in brightfield. Bar in (a) = 20 µm. (b), (d) Cells were photographed in brightfield shown in (a) and (b) |
5 Subcellular localization of CAX protein in Puccinellia tenuiflora
We cloned a Cation/H+ antiporter gene (PutCAX2) from Puccinellia tenuiflora. The recombinant plasmids of pBI121-GFP and pBI121- GFP-PutCAX2 were transformed into Arabidopsis mesophyll protoplast. Upper shows the expression of the GFP protein in Arabidopsis protoplasts, green fluorescence was distributed in cytoplasm; while in the GFP-PutCAX2 cells (below), green fluorescence was distributed on Golgi apparatus, which merged with the red fluorescence of Golgi-red dye (BODIPY® TR C5-ceramide complexed with BSA) (Figure 7). It indicates that PutCAX2 locates on the Golgi of Arabidopsis protoplas.
|
|
6 Conclusion
In this review, we showed the subcellular localization of different CAX proteins in different plants. The localization varies a lot from species to species. Both AtCAX1 and AtCAX2 are located on vacuolar membrane of Arabidopsis. Also, AtCAX4 and VCAX1p are located on vacuolar membrane of transgenic tobacco. But the difference is that GmCAX1 mainly resides on the plasma membrane of transgenic Arabidopsis and onion epidermal cells.
When expressed in mesophyll cell protoplast of Arabidopsis, OsCAX3 also resides on the plasma membrane. The interesting thing is that PutCAX2 from Puccinellia tenuiflora surprisingly locates on the Golgi of transgenic Arabidopsis. We still yet don’t know what makes all this difference, and what the key point is to clear the mystery behind. In the near future, it is important to combine the localization and the expression of the CAX genes in various growth and developmental mutants to explore the mechanism of CAX.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (NSFC, No. 31270311) awarded to Xinxin Zhang.
http://dx.doi.org/10.1104/pp.010857
Conn S.J., Gillham M., Athman A., Schreiber A.W., Baumann U., Moller I., Cheng N.H., Stancombe M.A., Hirschi K.D., Webb A.R., Burton R., Kaiser B.N., Tyerman S.D., Leigh R.A., 2011, Cell specific vacuolar calcium compartmentation regulates apoplastic calcium concentration, gas exchange and plant productivity, Plant Cell, 23: 240-257
Kendal D.H., Victor D.K., Nathaniel L.W., George J. W., 2000, Expression of Arabidopsis CAX2 in Tobacco, Altered Metal Accumulation and Increased Manganese Tolerance, Plant Physiology, 124: 125-133
Kost B.S., Spielhofer P., Chua NH., 1998, A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes, Plant J, 16: 393-401
Maeshima M., 1992, Characterization of the major and integral protein of vacuolar membrane, Plant Physiol, 98: 1248-1254
Manohar M., Mei H., Franklin A.J., Sweet E.M., Shigaki T., Riley B.B., MacDiarmid C.W., Hirschi K.D., 2010, Zebrafish (Danio rerio) endomembrane antiporter similar to a yeast cation ⁄H+ transporter is required for neural crest development, Biochemistry, 49: 6557-6566
Shimoni Y., Zhu X., Levanony H., Segal G., Galili G., 1995, Purification, characterization and intracellular localization of glycosylated protein disulfide isomerase from wheat grains, Plant Physiol., 108: 327-335
. PDF(868KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Hongzhong Chen
. Xinxin Zhang
Related articles
. CAX
. Antiporter
. Fluorescence
. Localization
. Organelle
Tools
. Email to a friend
. Post a comment