Studies on Construction of Regeneration System and Genetic Transformation of Puccinellia chinampoensis 
2. Asian Natural Environmental Science Center(ANESC), University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 188-0002, Japan
 Author
Author  Correspondence author
Correspondence author
Bioscience Methods, 2011, Vol. 2, No. 5 doi: 10.5376/bm.2011.02.0005
Received: 02 Jun., 2011 Accepted: 20 Jun., 2011 Published: 23 Jun., 2011
Wang et al., 2011, Studies on Construction of Regeneration System and Genetic Transformation of Puccinellia chinampoensis, Bioscience Methods, doi:10.5376/bm.2011.02.0005
The regeneration system was established and the genetic transformation was processed, using the mature seeds of Puccinellia chinampoensis as explants. The results showed that the callus induction rate could reach to 39.75% after 19days of induction with the altered mediumâ… (adding 4 mg/L 2,4-D, 500 mg/L proline and 500 mg/L glutamine) (pH 5.8~6.0). When using the modified medium S (adding 2 mg/L 2,4-D, 1 mg/L ABA, 500 mg/L proline and 600 mg/L caseinhydrolysate) (pH 5.8~6.0) as the subculture medium, we can get much more embryogenic callus. The differentiation rate could reach to 53.73% using the differentiation medium (MS as the basic medium, adding 0.4 mg/L ABA, 0.04 mg/L IAA, 500 mg/L proline and 600 mg/L caseinhydrolysate) (pH 5.8~6.0). On the genetic transformation, the transformation efficiency was best when the infect time lasted 30 min, making using of the Agrobacterium tumefaciens mediated pBI121-GUS transformation when OD600 reached to 0.8~1.0.
With the expansion of soil salinization globally, discovering the salt and alkali tolerance mechanisms of plants is the important evidence for improving the development and utilization of salty and alkali soil (Flowers et al., 2004). Puccinellia chinampoensis is a kind of pioneer plant growing well in the salt and alkali soil, and it has the other advantages such as low temperature tolerance and good palatability, so it is the excellent grass for pasturage. It has been screened out to manage the salinization since 1980s and have achieved good results in China (Wang et al., 2004). At the present time, researches of Puccinellia chinampoensis are focused on the structural anatomy, physiology ecology and molecular biology and so on. Separating functional genes related to salt and alkali stresses from salt plants is the key to discuss the molecular mechanism of plant salt tolerance and to breed the new vatieties of plants. Puccinellia chinampoensis is not only an excellent grass, but also a precious resource providing a wealth of salinity tolerance genes.
In this study, we discussed a series of culture conditions of callus induction, plant regeneration and genetic transformation of Puccinellia chinampoensis, which provided the technical support and theoretical basis for the further exploring the genetic improvement and cellular breeding engineer.
1 Results and Analysis
1.1 Impact on the callus induction of hormone 2,4-D
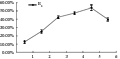
After 7~9 days’ culture on the induction medium, callus would be induced out at the base of embryo. It can reduce the induction time at the light cultivate condition, but at this time, the embryo will grow into a seedling shoot at low concentrations of 2,4-D, which can inhibit the induction of callus. To prevent the formation of seedling shoot, more 2,4-D was supplemented, and when it reached to 4 mg/L, the induction rate could be reached to 39.7% after 14 days’ cultivation (Table1; Figure 1). Additionally, the induction rate will increase as the prolonged induction time at a degree.
![]()
Table 1 Influence of different concentrations of 2,4-D on callus induction of seed after 14 days’ culture

Figure 1 Influence of different concentrations of 2,4-D on callus induction after 14 days’ culture
1.2 Influence of different basic medium on callus induction of seed
The different basic media, containing different nutrient contents and compositions, have obviously different effect on callus induction. On the MS medium supplemented with 4 mg/L 2,4-D, the callus induction of seed reaches to the summit (47.52%) (Table 2). However, the callus appeared differently on the different basic media. On the MS medium containing 4 mg/L 2,4-D, the callus induced out seemed white, texture soft and prone to have wet and sticky surface, which is also named water-like callus and can be sure of non-embryogenic callus. On the N6 medium containing 4 mg/L 2,4-D, the callus looks to be lightly yellow, texture loosen and have a dry surface, but less callus formed. However, in this study, using the altered medium I supplemented with 4 mg/L 2,4-D, we got the best result. The induced callus looks to be lightly yellow, texture compact, surface dry and have the granular appearance, which is thought to be embryogenic callus basically (Table 2 and Figure 2).
|
|
|
|
1.3 Impact of different media on callus subculture
The growth of callus was monitored to be distinctly different under different subculture media. Among these media, the growth rate of callus was the highest under the MS medium (11.69±3.24)%, followed by the altered medium S, and the worst on the medium N6 (9.65±2.87)%. Although the callus grew faster than others, it seemed to be loosen, friable and almost non-embryogenic. Callus on the N6 medium grew slower than that on the MS medium, but the callus had dense texture and almost is embryogenic. The growth of callus on the altered medium S was between that of MS and N6 (Table 3). Therefore, the altered medium S was chosen to be the basic subculture medium. MS medium contains a large number of ammonium nitrogen, which can stimulate the multiplication of callus cells, and N6 medium containing a lot of nitrate, which can be prone to form the dense texture to maintain the embryogenic callus. The modified medium S combines the advantages of the two above media, which can be more conducive to the formation of embryogenic callus and improve the quality of callus, useful to the differentiation of the next step.
|
|
1.4 Impact of hormone ABA on the callus subculture
Abscisic acid (ABA) is a plant hormone with high activities. It plays an important role in increasing induction rate and improving the quality of callus. Although a certain concentration of ABA can reduce callus growth rate, it is very useful to improve the status of callus to be conducive to the formation of embryogenic callus. Our studies indicated that the growth rate and the state of callus reached to the best, and it is prone to form the embryogenic callus with dense texture and dry appearance when the callus was cultivated on the medium supplemented 1 mg/L ABA (10.54±0.81)%. However, as the concentration of supplemented ABA increasing, the induced callus will grow to be brown and a large number of hairy roots will grow up (Table 4). Therefore, it is very useful for the callus growth to supplement 1 mg/L ABA into the subculture medium.
|
|
1.5 Effect of different hormone concentrations on the callus differentiation
In this study, we discussed the effects of different hormone combination ratios and hormone concentration supplemented into the differentiation medium. We found that when the hormone combination ratio (C6-BA: CIAA) is 10:1, different contents of hormone containing in the medium will have different effects on the callus differentiation. High concentration of hormone will negatively affect the differentiation, increase the browning rate, and even directly lead to the death of callus cells. When the concentrations of 6-BA and IAA supplemented is 0.4 mg/L and 0.04 mg/L, the callus differentiation rate reaches up to 53.73% (Table 5; Figure 3). To prevent browning, 500 mg/L proline was supplemented into the induction, subculture and differentiation media.
|
|
|
|
1.6 Infection time of Agrobacterium have an impact on the callus genetic transformation
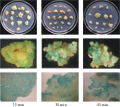
Infection time of Agrobacterium have an obvious effect on the transformation efficiency. Long time of infection will inhibit the growth of the receptor, hard to get the tranformants, but short time of infection go against the attachment of Agrobacterium to the receptor and decrease the transformation rate. In this study, the result showed that the transient expression efficiency of GUS gene by 15 minutes of infection is 31.43%, 30 minutes’ infection is 47.14% and 45 minutes’ infection is 27.14% (Table 6). As shown in Figure 4, the callus looked to be darker by GUS staining after 30 minutes and 45 minutes of infection than that of 15 min of infection. But when the callus was infected by Agrobacterium containing GUS gene 45 minutes, the transient expression efficiency was low (Table 6). These results indicate that 30 minutes of infection was appropriate to the transformation of recombinated Agrobacterium (OD600≈0.8~1.0).
|
|
|
|
2 Discussion
In summary, 2,4-D is a necessary regulator in the process of callus induction of gramneal plants (Mitsuoka et al., 1994). In this study, the highest induction rate we have got is 39.75% using the altered medium I supplemented with 4 mg/L 2,4-D as the induction medium (Table 1; Figure 1). Among the grass family, the callus induction of Puccinellia chinampoensis needs more 2, 4-D than others. Besides this regulator, the composed elements, such as C source (Lee et al., 2002), N source (Grimes and Hodges, 1990), amino acids (Ozawa and Komamine, 1989) (Chowdhry et al., 1993) and iron salts, can also affect the callus induction. Adjustment the contents of these elements can change the induction rate, as shown in Table 2 and Figure 2.
In the process of callus subculture, adding a certain amount of ABA can effectively improve the quality of callus (Higuehi and Maeda, 1990). In the callus induction and subculture process, the rational use of nitrate and ammonium nitrogen will be more beneficial to the formation of embryogenic callus (Ge et al., 2006), which was determined in this study where the modified medium S was chosen to be the best medium for the callus subculture (Table 3).
About the callus differentiation, proembryogenic masses (PEMs) in the embryogenic callus can gradually develop into somatic embryos at appropriate culture conditions, and further differentiate into a complete plant (Arnlod et al., 2002). However, non-embryogenic callus is composed by relatively bigger cells, which have large vacuoles but little cytoplasm, and there are less PEMs in the surface of callus. High proportion of cytokinin / auxin is more conducive to callus shoot differentiation, but if the hormone levels are too high, it will reduce the differentiation rate and increase the callus browning (Laukkanen et al., 1999), as shown in Table 5. A certain amount of proline added into the induction, subculture and differentiation media can suppress the callus browning (Tang and Newton, 2004).
In the callus genetic transformation test by Agrobacterium, the infection time is one of the primary factors that affect the transformation rate. Long time of infection will inhibit the growth of the receptor, hard to get the tranformants, but short time of infection go against the attachment of Agrobacterium to the receptor and decrease the transformation rate (Peng et al., 2005). To the callus transformation of Puccinellia chinampoensis in this study, 30 minutes was the best infection time by Agrobacterium containing GUS gene when the OD600 of the suspended liquid reached to 0.8~1.0 (Table 6; Figure 4).
3 Materials and Methods
3.1 Materials
Seeds of Puccinellia chinampoensi: Puccinellia seed will be soaked at 4℃ with distilled water for 4 to 7 days. After drying seeds, the seeds soaked in 75% alcohol 1 min, and wash 3 to 4 times with sterile water, and then soak them with 10% Sodium hypochlorite for 30 minutes, then rinse 3~5 times with sterile water, finally, dry them for later use in the clean bench.
Strains: Agrobacterium EHA105 containing plasmid PBI121-GUS (Agrrobcrcterum tumefaciens).
3.2 Callus induction conditions
On the basic medium(MS +500 mg / L proline +500 mg / L glutamine +30 g / L sucrose +0.8% agar (pH 5.8 ~ 6.0)), adding different concentrations of 2,4-D: 0 mg / L, 1 mg / L, 2 mg / L, 3 mg / L, 4 mg / L, 5 mg / L, 6 mg / L as the induction medium; On the basic medium (MS medium, N6 medium, altered medium I) (Mitsuoka et al., 1994), adding 4 mg / L 2,4-D, 500 mg / L proline, 500 mg / L glutamine, 30 g / L sucrose, 0.8% agar (pH 5.8 ~ 6.0) as the induction medium. After the disinfection of mature dry seeds , the callus were inoculated into induction medium, placed in (25 ± 10)℃, 80 μmol / (m2•s) light intensity, illumination time was 12 h / d for 2 weeks under the conditions.
3.3 Callus subculture conditions
On the basic medium(MS medium, N6 medium, modified medium S (Mitsuoka et al., 1994) respectively), adding 2 mg / L 2,4-D, 500 mg / L proline, 600 mg / L hydrolyzed casein, 30 g / L sucrose, 0.8% agar (pH 5.8 ~ 6.0) as a subculture medium; and then on the basic medium (S +2 mg / L 2,4-D +500 mg / L proline +600 mg / L +30 g casein hydrolyzate / L sucrose +0.8% agar (pH 5.8~6.0)), adding 0 mg / L, 1 mg / L and 2 mg / L ABA as a subculture medium. after subculturing , placed the plates under (25 ± 10)℃, 80 μmol / (m2•s) light intensity, illumination time was 12 h / d under the conditions of culture and subculture period of 20 d. In the course of subculture, we select the drier, more compact growth good yellow embryogenic callus, not the non-embryogenic callus.
3.4 The differentiation conditions of the callus
On the basic medium (MS +500 mg / L proline +600 mg / L hydrolyzed casein +30 g / L sucrose +0.8% agar (pH 5.8 ~ 6.0)), adding the mixture(cell division: the ratio of 10:1: auxin Add 6-BA 4 mg / L, 2 mg / L, 1 mg / L, 0.5 mg / L, 0.4 mg / L, 0.3 mg / L and IAA 0.4 mg / L, 0.2 mg / L, 0.1 mg / L, 0.05 mg / L, 0.04 mg / L, 0.03 mg / L respectively) as the differentiation medium, inoculated embryogenic callus twice subculturing, placed them under (25 ± 10)℃, 80 μmol / (m2 • s) light intensity, illumination time was 14 h / d.
3.5 Genetic transformation of the callus
The good growth embryogenic callus selected, afer preculturing, were then cultured and infected with the Agrobacterium suspension (OD600 of 0.8 to 1.0) containing PBI121-GUS plasmid.The time course was 15 min, 30 min and 45 min respectively. After that, co-cultured process was made in the medium for 7 d. Before GUS staining, in order to get rid of the bacteria, we filtrate the callus with vacuum pump. Finally, the efficiency of genetic transformation was measured under the different infection time.
Authors’ contributions
TW, XH and MQZ designed and conducted this experiments; XXZ and TT participated the experiment design and data analysis; SKL is the person who takes charge of this project, including experiment design, data analysis, writing and modifying of the manuscript. All authors have read and approved the final manuscript.
Acknowledgements
This work was supported by the Heilongjiang Provincial Program for Distinguished Young Scholars (JC200609) and State Forestry Administration 948 Program of PR China (No. 2008429) to Shenkui Liu. Authors appreciate two anonymous reviewers for their useful critical comments and revising advice to this paper. And also we mentioned some reagent suppliers and sequencing service providers in this work, that doesn’t mean we would like to recommend or endorse their products and services.
References
Arnlod S.V., Sabala I., Bozhkov P., Dyachok J., and Filonova L., 2002,Developmental pathways of somatic embryogenesis, Plant Cell Tiss. Org. Cult., 69(3): 233-249 doi:10.1023/A:1015673200621
Chowdhry C.N., Tyagi A.K., Maheshwari N., and Maheshwari S.C., 1993, Effect of L-proline and L-trytophan on somatic embryogenesis and plantlet regeneration of rice (Oryza sativa L. cv. Pusa 169), Plant Cell Tiss. Org. Cult., 32(3): 357-361
doi:10.1007/BF00042300
Flowers T.J., 2004, Improving crop salt tolerance, J. Exp. Bot., 55(396): 307-319 doi:10.1093/jxb/erh003 PMid:14718494
Ge X.J., Chu Z.H., Lin Y.J., and Wang S.P., 2006, A tissue culture system for different germplasms of indica rice, Plant Cell Rep., 25(5): 392-402 doi:10.1007/s00299-005-0100-7 PMid:16432631
Grimes H.D., and Hodges T.K., 1990, The inorganic NO3-/NH4+ ratio influences plant regeneration and auxin sensitivity in primary callus derived from immature embryos of indica rice (Oryza sativa L.), J. Plant Physiol., 136(3): 362-367
Higuehi N., and Maeda E., 1990, Enhaneed plant regeneration in rice callus cultures following abseisic aeid treatment, Japan J. Crop Sei., 59(2): 359-368
Laukkanen H., Haggman H., Kontunen-Soppela S., and Hohtola A., 1999, Tissue browning of in vitro cultures of Scots pine: role of peroxidase and polyphenol oxidase, Physiol. Plant, 106(3): 337-343 doi:10.1034/j.1399-3054.1999.106312.x
Lee K., Jeon H., and Kim M., 2002, Optimization of a mature embryo-based in vitro culture system for high-frequency somatic embryogenic callus induction and plant regeneration from japonica rice cultivars, Plant Cell Tiss. Org. Cult., 71(3): 237-244 doi:10.1023/A:1020305432315
Mitsuoka K., Honnda H., Xing X.H., and Unno H., 1994, Effect of intracellular 2,4-D concentration on plantlet regeneration of rice (Oryza sauva L.) callus, Appl. Microbiol. Biotechnol., 42(2-3): 364-366 doi:10.1007/BF00902743 doi:10.1007/s002530050264
Ozawa K., and Komamine A., 1989, Establishment of a system of high-frequency embryogenesis from long-term cell suspension cultures of rice (Oryza sativa L.). Theor. Appl. Genet., 77(2): 205-211 doi:10.1007/BF00266188
Peng H., Huang H.M., Yang Y.Z., Zhai Y., Wu J.X., Huang D.F., Lu T.G., 2005, Functional analysis of GUS expression patterns and T-DNA integration characteristics in rice enhancer trap lines, Plant Science, 168(6): 1571-1579 doi:10.1016/j.plantsci.2005.02.011
Tang W., and Newton R.J., 2004, Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.), Plant Science, 167(3): 621-628 doi:10.1016/j.plantsci.2004.05.024
Wang Z.C., Li Q.S., Li X.J., Song C.C., and Zhang G.X., 2004, Saline-alkali land management and countermeasures of sustainable agricultural development in Songnen Plain, Chinese Journal of Eco-Agriculture, 12(2): 161-163
. PDF(796KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Tao Wang
. Xue Han
. Mengqing Zhao
. Takano Tetsuo
. Shenkui Liu
Related articles
. Puccinellia chinampoensis
. Regeneration system
. Genetic transformation
Tools
. Email to a friend
. Post a comment