Research Article
Improved Production of Y-Decalactone from Castor Oil by UV Mutated Yeast Sporidiobolus salmonicolor (MTCC 485) 
2 Sri Krishnadevaraya university, Anantapur, Andhra pradesh, India
3 Jawaharlal technological university, Anantapur, Andhra pradesh, India
 Author
Author  Correspondence author
Correspondence author
Bioscience Methods, 2016, Vol. 7, No. 6 doi: 10.5376/bm.2016.07.0006
Received: 03 Mar., 2016 Accepted: 12 Dec., 2016 Published: 28 Dec., 2016
Nama S., Reddy L.V., Reddy B.V., Devanna N., and Rao D.M., 2016, Improved production of γ-Decalactone from castor oil by UV mutated yeast Sporidiobolus salmonicolor (MTCC 485), Bioscience Methods, 7(6): 1-9 (doi: 10.5376/bm.2016.07.0006)
γ-Decalactone (GDL; C10H18O2) is an industrially important flavor compound having peachy fruit aroma and approved by FDA as a food additive. The aim of the present study is to enhance the production of γ-Decalactone by mutant strain of Sporidiobolus salmonicolor MTCC 485through batch cultivation using castor oil as substrate. Mutation studies were carried out using UV light and four potential mutant strains were developed (UV1, UV2, UV3 and UV4). Bioconversion of castor oil for the production of γ-Decalactone by obligate aerobic yeast S. salmonicolor was investigated in a 5 l bioreactor. Four mutant strains were shoed fast growth and higher production of γ-Decalactone when compared to Wild strain which produced 62.2 mg/l. maximum at 96 h. Among the four strains selected UV3 was produced 81.9 mg/l maximally at 96 h. It was showed 33% higher production of γ-Decalactone when compared to the wild strain.
1 Introduction
Many microbial processes have been described able to produce interesting flavors, the number of industrial applications are limited. A reason for this in most cases is the low yield. The microbial flavors are often present only in low concentrations in the fermentation broths, resulting in high costs for upstream and down-stream processing. The development of Indigenous specific fermentation techniques and recovery methods is an important challenge for researchers in this field. The present investigation is a preliminary attempt to produce ingenious technology for the production of natural flavor.
γ-Decalactone (GDL; C10H18O2) is a well-known industrially important microbial aroma found in various foods and beverages (Iacazio et al., 2002). It is classified and generally recognized as a safe (GRAS) food additive by the U.S. Food and Drug Administration (New Hampshire, MD, USA), and used as a “natural” food flavoring additive when produced by biotechnological processes (Aguedo et al., 2004a). γ Lactones are widely distributed in nature; this moiety is present in around 10% of all natural compounds. Most display a broad biological profile including strong antibiotic, antihelmetic, antifungal, antitumour, antiviral, anti-inflammatory and cytostatic properties, which make them interesting lead structures for new drugs. An increasing demand for natural products has resulted in the use of biotechnological processes for the production of these lactones. This has led to numerous patents being taken out, and nowadays the biotechnologically produced lactone family is mainly represented by γ-Decalactone, but also to a smaller extent by γ-Dodecalactone and γ-Octalactone.
The world of flavors and fragrances has begun to industrialize with the increase in the demand of these substances. It can be easily obtained from hydroxyl fatty acids by microbial transformation and is also naturally present in many products fermented by yeasts. Most of the other lactones are also encountered in fermented products but are far more difficult to produce. Tahara et al. (1973) presented the first report on biotechnological GDL production using Sporidiobolus salmonicolor (Sporobolomyces odorus). Continuous investigations by researchers have increased the number of microorganisms that produce GDL, such as Pichia guilliermondii (Iacazio et al., 2002), Candida sp. (Aguedo et al., 2004b), Sporidiobolus salmonicolor (Lee et al., 1999), Sporobolomyces odorus (Berger & Drawert, 1985; Lee & Chou, 1994; Gatfield & Rabenhorst, 1999; Beney et al., 2001), Rhodotorula glutinous (Gatfield & Rabenhorst, 1999) and especially Yarrowia lipolytica (Waché et al., 2006). Previous studies focused mostly on three aspects of the production process: fermentation control and process optimization, including also optimization of nutritional and non-nutritional conditions (Berger & Drawert, 1985; Lee & Chou, 1994; Lin et al., 1996; Gatfield & Rabenhorst, 1999; Lee et al., 1999; Beney et al., 2001; Aguedo et al., 2004b), and ultra structural research with the objective to determine the metabolism responsible for bioconversion to GDL through peroxisomal or mitochondrial β-oxidation (Blin-Perrin et al., 2000; Feron et al., 2005); and the β-oxidation pathway and its optimization (Waché et al., 2001). Recently, Gomes et al. (2013) studied the impact of lipase hydrolysis of castor oil on GDL, providing a new way to improve GDL production and speed up the biotransformation process. During the bioconversion of ricinoleic acid to g-decalactone under controlled pH conditions, Sporidiobolus salmonicolor produced only the lactone form, while Sporidiobolus ruinenii produced both the lactone form and a precursor. The effects of some physical factors, including preculture time, media initial pH and bead concentrations, on the production of GDL by immobilized Sporidiobolus salmonicolor CCRC 21975 within calcium alginate beads were investigated. In the present study we have studied the development of UV mutant strain for the improved production of GDL Sporidiobolus salmonicolor (MTCC 485).
2 Materials and Methods
2.1 Microorganism
Sporidiobolus salmonicolor (MTCC 485) was obtained from the Microbial Type Culture Collection and Gene Bank, Institute of Microbial Technology (IMTECH, Chandigarh), India and was maintained in YM Agar media slants at 25°C. After three days growing yeast spores were stored in refrigerator (Figure 1).
|
Figure 1 The Yeast Sporidiobolus salmonicolor (MTCC 485) on YM Agar media slants at 25°C |
2.2 Culture conditions
Pre cultures were prepared by transferring a loopful of microorganisms grown on YM agar slants to 100 ml preculturing glucose medium (glucose:15, tryptone:0.5, yeast extract:1 malt extract:1 casaminoacids:2 K2HPO4: 2 Cacl2: 0.13, Feso4: 0.01 and MgSO4: 3 g/l) in 250-ml Erlenmeyer flasks and then incubated in shaking incubator at 160 rpm and at 25°C for various cultivation periods (16, 24 or 42 h). To study the effects of operational parameters on GDL production with stationary phase cells, these were supplemented with 0.1% methyl Recinoleate (castor oil) in 250-ml screw-capped Erlenmyer flasks which were incubated at 25°C on a rotary shaker at 160 rpm. The medium initial pH was adjusted to 4.0, 5.0, 6.0, 7.0 and 8.0 by titration with sterilized 1 M HCl and 1 MNaOH.
2.3 Mutagenesis and mutant isolation
For the analysis of survival rates by UV mutagenesis, cells grown on YM agar slants for 18 hours at 25°C. After incubation culture was collected and suspended in sterile distilled water. After that cell concentration was determined by counting cells was spread on YM agar plates. The plates were placed under a UV lamp at a distance of 55 cm and were irradiated for various periods of time (3, 5, 7, 10 min). Following irradiation, the plates were kept in dark for 1 hour before incubation at 25°C for 3 days. The number of colonies on plates was then counted to determine survival rates. Mutant preculture was transferred into 250 ml screw-capped flask which contain 100 ml Glucose medium incubated with shaking at 160 rpm and at 25°C. Cells were precultured in a Glucose medium for 49 hours to the logarithmic phase (Figure 2).
|
Figure 2 250 ml screw-capped Erlenmeyer flask which contain Glucose medium |
2.4 Fermentation studies
2% (v/v) of a Yeast suspension (from about 1 to 10 × 10 7 cells /ml) from the previously cultivated organism was inoculated to the 5 litre Fermentor which contain 2 litre fermentation media (Glucose medium). The cultures were agitated (or) stirred at 250 rpm. In the fermentor experiments, the cultures were aerated (1 vvm) with a KLa of 90/ hr. When cells reached the stationary phase, the desired volume of methyl ricinoleate (Castor oil) was added to the medium to initiate the bioconversion process. Gas was monitored during the entire fermentation: Oxygen and Carbon dioxide concentration was monitored using Servomen 1 100 gas analyser and URAS 3G analyser respectively (Figure 3). Fermentation process was carried out for various time periods such as 24, 48, 72, 96, 120 hours respectively. Fermentation process is carried out for various pH values like 7, 6, 5, 4, 3, by addition of NaOH (2.5N) or H2S04 (2.5N) in 500 mL culture flasks
|
Figure 3 New Brunswick BIO FLO 110 lab scale fermentor used for decalactone production |
3 Analytical procedures
3.1 Dry weight and viability
Viability 1 ml of the culture was diluted a million-fold in a sterile 0.85% NaCl solution. After vortex mixing, 100~1 of each dilution was plated onto Petridishes filled with the medium described above supplemented with agar (20 g/c) (Figure 4). Cell colonies were counted at different times. Dry weight 5 ml of the culture was filtered under vacuum and washed first with a mixture of ethanol/acetone (50:50, v/v) following by a second wash with distilled water only. The filtered biomass was put in small aluminum dishes and dried at 70°C until the weight became constant.
|
Figure 4 Petridishes filled with the medium described above supplemented with agarc |
3.2 Extraction and aroma analysis
The fermentation liquid was micro filtered (0.2 µm) and the retentate (the residue containing the product and the biomass) was extracted batch-wise with eathanol. The product was neutralized by means of aqueous NaOH and NaCl was added under agitation .An upper phase (containing the product) was separated and was extracted with MTBE (methyl tri butyl either) After solvent evaporation, the extract was flash distilled under vacuum. The distillate consisted of 40% of γ–Decalactone. Gas chromatography (Agilent 7890) Volatile compounds were analyzed on a DB-FFAP bonded fused silica capillary column (30 m, 0.32 mm id, 0.25 pm film). Injection (1~1) was splitless/split (30 s). The temperature was raised from 40 to 240°C at 3°C/min. The hydrogen carrier gas velocity was 50 cm/s at room temperature. Integration of the lactone GC peaks was carried out using a chemstation software.
3.3 Growth behavior and production of GDL by S. Salmonoicolor T
The growth of S. salmonicolor in fermenter supplemented with castor oil hydrolysate containing 0.06% ricinoleic acid at the start of batch cultivation is shown in Figure 5. The population of S. salmonicolor increased from approximately 4.3 x 104 CFU/ml at the start of cultivation to approximately 1.7 x 108 CFU/ml at 72 h of cultivation at which time the culture entered the stationary growth phase. Thereafter, the population remained constant at approximately 1.8 x 108 CFU/ml up to 120 h of cultivation in the batch culture.
|
Figure 5 The growth of S. salmonicolor in fermenter supplemented with castor oil hydrolysate containing 0.06% ricinoleic acid at the start of batch cultivation |
The yield of γ-Decalactone increased rapidly after 24 h of cultivation and reached a maximum of 62.2 mg/l after 96 h up to 110 h. It was also observed that production rate will decreed after 110 h.
4 Effect of Mutation
After UV mutagenesis of Sporidiobolus salmonicolor for different time intervals (UV-1, UV-2, UV-3, UV-4) were used for production of γ-Decalactone in fermentation of glucose medium using castor oil. UV-3 producing 81.9 mg/l after 96 h, it was the maximum and high yield copared to wild and other mutants developed in the study. It was also observed that UV-1, UV-2mutants showed slight increment in production when compared to Wild type strain. And UV-4 mutant showed less GDL production when compared to all strains (Table 1; Figure 6).
|
Table 1 γ-decalactone concentration (mg/l) values for Wild type and mutant Strains with respective time Note: Yield of γ-decalactone during the cultivation of Sporidiobolus salmonicolor; (Wild type) and mutant strains UV-1, UV-2, UV-3, UV-4. Standard deviations are represented by the error bars |
|
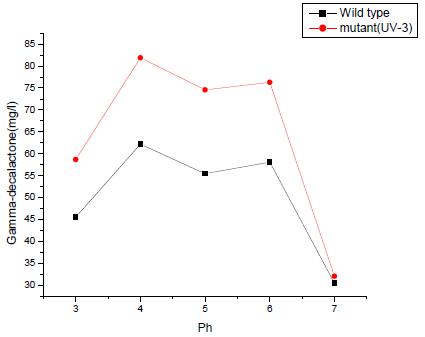
Figure 6 Production of γ-Decalactone by Sporidiobolus salmonicolor with pH of culture medium controlled at various levels. Standard deviations are represented by the error bars |
5 Effects of PH
Among the various pH-controlled conditions we tested, the greatest yield of γ-decalactone for Wild type and mutant strain (UV-3). For Wild type strain 62.1 mg/l of decalactone was detected in the medium maintained at pH 4.0. However, pH 6 also showed favorable conditions for decalactone production. For mutant strain (UV-3) 80.1 mg/l of decalactone was detected in the medium maintained at pH 4.0. It was also observed pH 5, pH 6 also show favorable conditions for decalactone production (Table 2).
|
Table 2 γ-decalactone concentration (mg/l) values for Wild type and mutant (UV-3) at various PH values |
6 Conclusion
In this study the process standardization of microbial production of lactone and strain improvement for g- Decalactone production by UV mutagenesis. Comparing between mutant strains (UV-1, UV-2, UV-3, and UV-4) obtained from the UV treatments UV-4 ability was less than that of the mutant strain (UV-1, UV-2, UV-3). UV-1 and UV-2 was not much affected by the UV treatment and UV-3 show best result in the maximum production of the g -Decalactone.
Comparing between wild type strain and mutant strain, mutant strain ability was not much affected to the PH tolerance that the wild type. Although the UV treatment was not as a promising tool for improving PH tolerance at PH 7, 6, and 5. It might useful for the improving the production of the g -Decalactone. In this study even if the random mutagenesis method as UV show high efficiency for the general strain improvement, but not show tolerance at pH 7, 6, 5.
7 Discussion
The present paper deals with studies on upstream fermentation process for the production of optically active γ-hydroxydecanoic acid which may optionally be converted by lactonization to γ-Decalactone. Depending on the embodiment of the invention employed, the fermentation process involves culturing or incubating a S. salmonicolor capable of hydrolyzing castor oil and effecting β-oxidation of the resulting hydrolysate. The use of castor oil or castor oil hydrolysate as the substrate is determined by the S. salmonicolor employed in the process. S. almonicolor was subjected to UV Mutations in order to increase the yield of the process.
The metabolism of ricinoleic acid by some Candida strains was investigated by Okui et al. (J. Biochemistry, 54,536-540, 1963) who showed that γ-hydroxydecanoic acid was an intermediate in the oxidative degradation of ricinoleic acid. However, only trace amounts of γ-hydroxydecanoic acid were recovered from the fermentation medium due to the metabolysis of γ-hydroxydecanoic acid upon completion of the fermentation.
Acknowledgements
DMR is highly thankful to the authorities of SK University, Anantapur, APNLBT, IISC, Bangalore and IMTECH, Chandigarh for providing cultures.
Aguedo M, Beney L, Waché Y, Belin J-M, and Gervais P., 2002, Interaction of odorous lactones with phospholipids: implications in toxicity towards producing yeast cells, Biotechnology Letters, 24:1975-1979
https://doi.org/10.1023/A:1021129800080
Aguedo M, Beney L, Waché Y, and Belin JM., 2003a, Interaction of an odorant lactone with model phospholipid bilayers and its strong fluidizing action in yeast membrane, International journal of Food Microbiology, 80:211-215
https://doi.org/10.1016/S0168-1605(02)00150-2
Aguedo M, Beney L, Waché Y, and Belin JM., 2003b, Mechanisms underlying the toxicity of lactone aroma compounds towards the producing yeast cells, Journal of Applied Microbiology, 94:258-265
https://doi.org/10.1046/j.1365-2672.2003.01828.x
Aguedo M, Gomes N, Escamilla Garcia E, Waché Y, Mota M, Teixeira JA, and Belo I., 2005, Decalactones production by Yarrowia lipolytica under increased O2 transfer rates, Biotechnology Letters, 27:1617-1621
https://doi.org/10.1007/s10529-005-2517-z
Aguedo M, Waché Y, Mazoyer V, Sequeira-Le Grand A, and Belin JM., 2003c, Increased electron donor and electron acceptor characters enhance the adhesion between oil droplets and cells of Yarrowia lipolytica as evaluated by a new cytometric assay, Journal of Agricultural Food Chemistry, 51:3007-3011
https://doi.org/10.1021/jf020901m
Alchihab M, Destain J, Aguedo M, Majad L, Ghalfi H, Wathelet JP, and Thonart P., 2009, Production of γ-decalactone by a psychrophilic and a mesophilic strain of the yeast Rhodotorula aurantiaca, Applied Biochemistry and Biotechnology, 158:41-50
https://doi.org/10.1007/s12010-008-8297-x
Alchihab M, Destain J, Aguedo M, Wathelet JP, and Thonart P., 2010, The utilization of gum tragacanth to improve the growth of Rhodotorula aurantiaca and the production of γ-decalactone in large scale, Applied Biochemistry and Biotechnology, 162:233- 241
https://doi.org/10.1007/s12010-009-8739-0
Alimardani P, Regnacq M, Moreau-Vauzelle C, Ferreira T, Rossignol T, Blondin B, and Berges T., 2004, SUT1-promoted sterol uptake involves the ABC transporter Aus1 and the mannoprotein Dan1 whose synergistic action is sufficient for this process, Biochemistry Journal, 381:195-202
https://doi.org/10.1042/BJ20040297
Alonso F, Oliveira E, Dellamora-Ortiz G, and Pereira-Meirelles F., 2005, Improvement of lipase production at different stirring speeds and oxygen levels, Brazilian Journal of Chemical Engineering, 22:9-18
https://doi.org/10.1590/S0104-66322005000100002
Alphand V, Carrea G, Wohlgemuth R, Furstoss R, and Woodley JM., 2003, Towards large-scale synthetic applications of Baeyer-Villiger monooxygenases, Trends Biotechnology, 21:318-323
https://doi.org/10.1016/S0167-7799(03)00144-6
Amaral P, Rocha-Leao M, Marrucho I, Coutinho J, and Coelho M., 2006, Improving lipase production using a perfluorocarbon as oxygen carrier, Journal of Chemical Technology Biotechnology, 81:1368-1374
https://doi.org/10.1002/jctb.1478
Amaral PF, Freire MG, Rocha-Leao MH, Marrucho IM, Coutinho JA, and Coelho MA., 2008, Optimization of oxygen mass transfer in a multiphase bioreactor with perfluorodecalin as a second liquid phase, Biotechnology and Bioengineering, 99:588-598
https://doi.org/10.1002/bit.21640
Ates S, Dingil N, Bayraktar E, and Mehmetoglu U., 2002, Enhancement of citric acid production by immobilized and freely suspended Aspergillus niger using silicone oil, Process Biochemistry, 38:433- 436
https://doi.org/10.1016/S0032-9592(02)00152-8
Bar R., 1989, Cyclodextrin-aided bioconversions and fermentations, Trends Biotechnology, 7:2-4
https://doi.org/10.1016/0167-7799(89)90070-X
Bartlett K, Eaton S., 1994, Intermediates of mitochondrial beta- oxidation, Biochemistry Society of Translation, 22:432-436
https://doi.org/10.1042/bst0220432
Bartlett K, Hovik R, Eaton S, Watmough NJ, and Osmundsen H., 1990, Intermediates of peroxisomal beta-oxidation, Biochemistry Journal, 270: 175-180
https://doi.org/10.1042/bj2700175
Bel I, Pinheiro R, and Mota M., 2005, Morphological and physiological changes in Saccharomyces cerevisiae by oxidative stress from hyperbaric air, Journal of Biotechnology, 115:397-404
https://doi.org/10.1016/j.jbiotec.2004.09.010
Belin JM, Dumont B, and Ropert F., 1994, Procédé de fabrication, par voie enzymatique, d'arômes, notamment des ionones et des aldéhydes en C6 à C10, Patent WO 94/08028
Blin-Perrin C, Molle D, Dufossé L, Le-Quéré J-L, Viel C, Mauvais G, and Féron G., 2000, Metabolism of ricinoleic acid into γ-decalactone: beta-oxidation and long chain acyl intermediates of ricinoleic acid in the genus Sporidiobolus sp, FEMS Microbiology Letters, 188:69-74
https://doi.org/10.1016/s0378-1097(00)00212-3
Bonnarme P, Djian A, Latrasse A, Féron G, Giniès C, Durand A, and Le Quéré J-L., 1997, Production of 6-pentyl-alpha-pyrone by Trichoderma sp. from vegetable oils, Journal of Biotechnology, 56:143-150
https://doi.org/10.1016/S0168-1656(97)00108-9
Boualem K, Waché Y, Garmyn D, Karbowiak T, Durand A, Gervais P, and Cavin J-F., 2008, Cloning and expression of genes involved in conidiation and surface properties of Penicillium camemberti grown in liquid and solid cultures, Research Microbiology, 159:110-117
https://doi.org/10.1016/j.resmic.2007.10.004
Bourel G, Nicaud JM, Nthangeni B, Santiago-Gomez P, Belin JM, and Husson F., 2004, Fatty acid hydroperoxide lyase of green bell pepper: cloning in Yarrowia lipolytica and biogenesis of volatile aldehydes, Enzyme Microbial Technology, 35:293-299
https://doi.org/10.1016/j.enzmictec.2003.12.014
Broun P, Shanklin J, Whittle E, and Somerville C., 1998, Catalytic plasticity of fatty acid modification enzymes underlying chemical diversity of plant lipids, Science, 282:1315-1317
https://doi.org/10.1126/science.282.5392.1315
Burfield T., 2010, 'Banned' essential oils'. Compiled from a variety of cropwatch sources, July 2010 v1.08.
Cardillo R, Fuganti C, Barbeni M, and Allegrone G., 1993, Procédé pour la préparation de delta lactones saturées par biohydrogénation des composés insaturés naturels correspondants à l'aide de micro- organisms, Patent EP O577463A2
Cardillo R, Fuganti C, Barbeni M, Cabella P, Guerda P, and Allegrone G., 1991, Procédé de production microbiologique des γ-et delta-lactones, Patent EP0412880
de Groot PW, Kraneveld EA, Yin QY, Dekker HL, Gross U, Crielaard W, de Koster CG, Bader O, Klis FM, and Weig M., 2008, The cell wall of the human pathogen andida glabrata: differential incorporation of novel adhesin-like wall proteins, Eukaryotic Cell, 7:1951-1964
https://doi.org/10.1128/EC.00284-08
Dufossé L, Feron G, Mauvais G, Bonnairme P, Durand A, and Spinnler H., 1998, Production of γ-decalactone and 4-hydroxy- decanoic acid in the genus Sporidiobolus, Journal of Fermentative Bioengineering, 86:169-173
https://doi.org/10.1016/S0922-338X(98)80056-1
Dufossé L, Souchon II, Feron G, Latrasse A, and Spinnler HE., 1999, In situ detoxification of the fermentation medium during γ-decalactone production with the yeast Sporidiobolus salmoni- color, Biotechnology Progress, 15:135-139
https://doi.org/10.1021/bp980113a
Eaton S, Bartlett K, and Quant PA., 2001, Carnitine palmitoyl transferase I and the control of beta-oxidation in heart mitochondria, Biochemistry Biophysics Research Community, 285:537-539
https://doi.org/10.1006/bbrc.2001.5201
Eaton S, Bursby T, Middleton B, Pourfarzam M, Mills K, Johnson AW, and Bartlett K., 2000, The mitochondrial trifunctional protein: centre of a beta-oxidation metabolon?, Biochemistry Society of Translation, 28:177-182
https://doi.org/10.1042/bst0280177
Endrizzi A, Pagot Y, Le Clainche A, Nicaud J-M, and Belin J-M., 1996, Production of lactones and peroxisomal beta-oxidation in yeasts, Critical Reviews in Biotechnology, 16:301-329
https://doi.org/10.3109/07388559609147424
Escamilla García E, Aguedo M, Gomes N, Choquet A, Belo I, Teixeira J, Belin J, and Waché Y., 2009, Production of 3-hydroxy-γ-decalactone, the precursor of two decenolides with flavouring properties, by the yeast Yarrowia lipolytica, Journal of Molecular Catalyst, Biology, 57:22-26
Escamilla García E, Belin J-M, and Waché Y., 2007a, Use of a Doehlert factorial design to investigate the effects of pH and aeration on the accumulation of lactones by Yarrowia lipolytica, Journal of Applied Microbiology, 103:1508-1515
https://doi.org/10.1111/j.1365-2672.2007.03379.x
Escamilla García E, Nicaud J-M, Belin J-M, and Waché Y., 2007b, Effect of acyl-CoA oxidase activity on the accumulation of γ-decalactone by the yeast Yarrowia lipolytica: a factorial approach, Biotechnology Journal, 2:1280-1285
https://doi.org/10.1002/biot.200700085
Escamilla García E., 2008, Aspects de la degradation de substrats hydrophobes en composés d'arômes par la levure Yarrowia lipolytica, PhD Thesis Université de Bourgogne
Farbood M, Harris G, Kizer LE, McLean LB, and Morris J., 1999, Process for preparing saturated lactones, products produced there from and organoleptic uses of said products, Patent EP0952226
Farbood M., 1991, Octalactone-containing composition, fermentation process for producing same and organoleptic uses thereof, Patent US719154
Feron G, Dufossé L, Mauvais G, Bonnarme P, and Spinnler HE., 1997, Fatty acid accumulation in the yeast Sporidiobolus salmonicolor during batch production of γ-decalactone, FEMS Microbiology Letters, 149:17-24
https://doi.org/10.1016/S0378-1097(97)00048-7
Feron G, Dufosse L, Pierard E, Bonnarme P, Le Quere JL, and Spinnler HE., 1996, Production, identification, and toxicity of γ-decalactone and 4-hydroxydecanoic acid from Sporidiobolus spp, Applied Environmental Microbiology, 62:2826-2831
Feron G, and Waché Y., 2005, Microbial biotechnology of food flavor production In: Dominick T (ed), Food biotechnology, 2nd edn. Dekker, New York, pp 407-441
https://doi.org/10.1201/9781420027976.ch1.16
Fraatz MA, Berger RG, and Zorn H., 2009, Nootkatone—a biotechnolog- ical challenge, Applied Microbiology Biotechnology, 83:35-41
https://doi.org/10.1007/s00253-009-1968-x
Freitas C, and Teixeira J., 2001, Oxygen mass transfer in a high solids loading three-phase internal-loop airlift reactor, Chemical Engineering Journal, 84:57-61
https://doi.org/10.1016/S1385-8947(00)00274-6
Gatfield IL, Güntert M, Sommer H, and Werkhoff P., 1993, Some aspects of the microbiological production of flavor-active lactones with particuliar reference to γ-decalactone, Chemical Mikrobiology Technology Lebensm, 15:165-170
Gatfield IL., 1999, Biotechnological production of natural flavor materials. In: Teranishi R, Wick EL, Hornstein I (eds) Flavor chemistry, thirty years of progress, Kluwer Academic, Plenum Publishers, New York, pp 211-227
https://doi.org/10.1007/978-1-4615-4693-1_19
Gomes N, Aguedo M, Teixeira JA, and Belo I., 2007, Oxygen mass transfer in a biphasic medium: influence on the biotransformation of methyl ricinoleate into g-decalactone by the yeast Yarrowia lipolytica, Biochemical Engineering Journal, 35:380-386
https://doi.org/10.1016/j.bej.2007.02.002
Gómez-Díaz D, Gomes N, Teixeira J, and Belo I., 2009, Oxygen mass transfer to emulsions in a bubble column contactor, Chemical Engineering Journal, 152:354-360
https://doi.org/10.1016/j.cej.2009.04.059
Gómez-Díaz D, Gomes N, Teixeira J, and Belo I., 2010, Gas-liquid interfacial area in the oxygen absorption to oil-in-water emul- sions in an airlift reactor, Canidian Journal of Chemical Engineering, 88:561-564
Groguenin A, Waché Y, Escamilla Garcia E, Aguedo M, Husson F, LeDall M, Nicaud J, and Belin J., 2004, Genetic engineering of the beta- oxidation pathway in the yeast Yarrowia lipolytica to increase the production of aroma compounds, Journal of Molecular Catalyst Biology, 28:75-79
https://doi.org/10.1016/j.molcatb.2004.01.006
Hall CE, Husson F, and Kermasha S., 2004, Characterization of an enriched lipoxygenase extract from Aspergillus niger in terms of specificity and nature of flavor precursors production, Jornal of Molecular Catalyst Biology, 29:201-209
https://doi.org/10.1016/j.molcatb.2003.11.013
Husson F, Bompas D, Kermasha S, and Belin JM., 2001, Biogeneration of 1-octen-3-ol by lipoxygenase and hydroperoxide lyase activities of Agaricus bisporus, Process Biochemistry, 37:177-182
https://doi.org/10.1016/S0032-9592(01)00201-1
Ju L.K, and Chase G., 1992, Improved scale-up strategies of bioreactors, Bioprocess Bioengineering, 8:49-53
https://doi.org/10.1007/BF00369263
Kataoka M, Honda K, Sakamoto K, and Shimizu S., 2007, Microbial enzymes involved in lactone compound metabolism and their biotechnological applications, Applied Microbiology and Biotechnology, 75: 257-266
https://doi.org/10.1007/s00253-007-0896-x
Lange H, and Garbe L., 2000, Yeast alpha-oxidation enzymes, used to degrade organic compounds by one carbon atom, used to convert 5-hydroxydecanoic acid to γ-nonalactone, Patent DE19929577
Lee S, Cheng H, Chen W, and Chou C., 1998, Production of γ-decalactone from ricinoleic acid by immobilized cells of Sporidiobolus salmonicolor, Process Biochemistry, 33:453-459
https://doi.org/10.1016/S0032-9592(98)00013-2
Lee S, Cheng H, Chen W, and Chou C., 1999, Effect of physical factors on the production of g-decalactone by immobilized cells of Sporidiobolus salmonicolor, Process Biochemstry, 34:845-850
https://doi.org/10.1016/S0032-9592(99)00010-2
Lee S, Lin S, and Chou C., 1995, Growth of and production of g- decalactone by Sporobolomyces odorus in jar fermentors as affected by pH, aeration and fed-batch technique, Journal of Fermentation Bioengineering, 80:195-199
https://doi.org/10.1016/0922-338X(95)93219-A
Ly MH, Cao Hoang L, Belin J-M, and Waché Y., 2008, Improved co- oxidation of beta-carotene to beta-ionone using xanthine oxidase- generated reactive oxygen species in a multiphasic system, Biotechnology Journal, 3:220-225
https://doi.org/10.1002/biot.200700064
Mlícková K, Roux E, Athenstaedt K, D'Andrea S, Daum G, Chardot T, and Nicaud J.M., 2004, Lipid accumulation, the formation of lipid bodies, and acyl-CoA oxidases of the yeast Yarrowia lipolytica, Applied Environmental Microbiology, 70:3918-3924
Nago H, Matsumoto M, and Nakai S., 1993, 2-deceno-delta-lactone- producing fungi, strains of Fusarium solani, isolated by using a medium containing decano-delta-lactone as the sole carbon source, Bioscience, Biotechnology and Biochemistry, 57:2107-2110
https://doi.org/10.1128/AEM.70.7.3918-3924.2004
Okui S, Uchiyama M, and Mizugaki M., 1963, Metabolism of hydroxy fatty acids: 1. Metabolic conversion of ricinoleic acid by a certain microorganism to 8-D-(+)-hydroxy tetradec-cis-5-enoic acid, Journal of Biochemistry, 53:265-270
Osumi M, Fukuzumi F, Yamada N, Nagatani T, Teranishi Y, Tanaka A, and Fukui S., 1975, Surface structure of some Candida yeast cells grown on n-alkanes, Journal of Fermentation Technology, 53:244-248
Pagot Y, Le Clainche A, Nicaud J-M, Waché Y, and Belin J-M, 1998, Peroxisomal beta-oxidation activities and γ-,ecalactone production by the yeast Yarrowia lipolytica, Applied Microbiol Biotechnology, 49:295-300
https://doi.org/10.1007/s002530051172
Picataggio S, Deanda K, and Dudley E., 1993, Site-specific modification of the Candida tropicalis genome, Patent US5254466 (A) Rabenhorst J, Gatfield I., 2000, Process for the production of γ-decalactone, Patent WO0024920
Rali T, Wossa S, and Leach D., 2007, Comparative chemical analysis of the essential oil constituents in the bark, heartwood and fruits of Cryptocarya massoy (Oken) Kosterm. (Lauraceae) from Papua New Guinea, Molecules, 12:149-154
https://doi.org/10.3390/12020149
Souchon I, Spinnler HE, Dufossé L, and Voilley A., 1998, Trapping of γ-decalactone by adsorption on hydrophobic sorbents: application to the bioconversion of methyl ricinoleate by the yeast Sporidiobolus salmonicolor, Biotechnology Techchniques, 12:109-113
https://doi.org/10.1023/A:1008880231677
Ta TMN, Cao-Hoang L, Lourdin M, Phan-Thi H, Goudot S, Marechal PA, and Waché Y., 2010a, A shift to 50°C provokes death in distinct ways for glucose- and oleate-grown cells of Yarrowia lipolytica, Applied Microbiology and Biotechnology (in revision)
Van Roermund CW, Waterham HR, Ijlst L, and Wanders R.J., 2003, Fatty acid metabolism in Saccharomyces cerevisiae, Cell Molecular Life Science, 60:1838-1851
https://doi.org/10.1007/s00018-003-3076-x
Waché Y., 2010, Production of dicarboxylic acids and flavours by the yeast Yarrowia lipolytica. In: Barth G, Steinbüchel A (eds) Yarrowia lipolytica, molecular biology and biotechnology, in press. Springer, Berlin
Wang H, Le Dall M-T, Waché Y, Laroche C, Belin J-M, and Nicaud J-M., 1999b Cloning, sequencing and characterization of five genes coding for Acyl-CoA oxidase isozymes in the yeast Yarrowia lipolytica, Cell Biochemistry and Biophysics, 31:165-74
https://doi.org/10.1007/BF02738170
Zelena K, Hardebusch B, Hulsdau B, Berger RG, and Zorn H., 2009, Generation of norisoprenoid flavors from carotenoids by fungal peroxidases, Journal of Agricultural Food Chemistry, 57:9951-9955
https://doi.org/10.1021/jf901438m
Zorn H, Langhoff S, Scheibner M, and Berger RG.,2003a, Cleavage of beta, beta-carotene to flavor compounds by fungi, Applied Microbiology and Biotechnology, 62:331-336
. PDF(633KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. S. Nama
. L.V. Reddy
. B.V. Reddy
. N. Devanna
. D.M. Rao
Related articles
. γ-Decalactone
. Sporidiobolus salmonicolor
. Castor oil
. UV mutation
. Medium pH
Tools
. Email to a friend
. Post a comment
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)


