 Author
Author  Correspondence author
Correspondence author
Bioscience Methods, 2012, Vol. 3, No. 4 doi: 10.5376/bm.2012.03.0004
Received: 02 Feb., 2012 Accepted: 25 May, 2012 Published: 08 Jun., 2012
Zhang et al., 2012, Factors Affecting Regeneration of Tomato Cotyledons, Bioscience Methods, Vol.3, No.4 27-33 (doi: 10.5376/bm.2012.03.0004)
In this research, we employed the tomato cultivar named Zhongshu Si Hao as research material to study the influences on the regeneration of adventitious buds under the different conditions with hormone combinations, various explants, different parts of cotyledons and cotyledon inoculatingmethods; we also screened the best method for seed surface disinfection and the most appropriate medium for the rooting of adventitious buds. The results showed that the best procedures for surface disinfection of tomato seeds would be as follows: firstly, immersing the seeds in 70% ethanol for 30 second, then rinsing them in 20% NaClO with tween-20 for 20 min. The most ideal explant for inducing buds is the middle part of the cotyledon, followed by the stem and the leaf. the best medium formula for cotyledon regeneration is MS+ZT 2 mg/L+IAA 0.1 mg/L, and placing the cotyledon upside down is better to induce the adventitious buds than that placing upside. The best medium for rooting of adventitious buds is 1/2MS+IAA 0.3 mg/L. This research would lay a foundation establish transformation system of tomato in our future research.
Tomato (lycopersicum esculentum Mill), an annual or perennial plant, belongs to Lycopersicon genus in the Solanaceae family; the tomato has become a worldwide fruit and vegetable due to its beautiful appearance, fragrant flavor, nutrition and high economic value, especially in recent years with the discovery of lycopene, anti-oxidation and anti-cancer function, edible benefit and economic value of the tomato are received more attentions (Liang and Wang, 2003, Xinjiang Petroleum College of Education, 7(2): 28-30, 34).
In production, the tomato often suffered from the pathogen infections of blight, fusarium wilt, gray mold, late blight and early blight infection; some varieties have little resistance resulting in yield decrease. In recent years, the great success is achieved in genetically engineered resistant gene transferred to plants, these technologies have also become an important means in tomato breeding program (Gao, 2006).
At present, although it has been reported that the exogenous gene could be introduced into the tomato genome by pollen tube transformation (Sanford et al., 1985; Huang et al., 1997; Tian et al., 2000), the most popular and mature technology applying in the genetic transformation of tomato should be Agrobacterium-mediated method. A variety of exogenous genes have been successfully tranferred into tomato by using Agrobacterium-mediated method (Pozueta-Romero et al., 2001; Van Roekel at al., 1993; Frary and Earle, 1996).
However, the efficiency of Agrobacterium-mediated transformation of tomato is known to be pretty low. It the process of tomato genetic transformation, the average efficiency of the cotyledon transformation was only 7.6% based on several transformation tests with triparental mating Agrobacterium (Ye, 1994); it was reported that the regeneration frequency of cotyledon for transforming ACC synthase gene and its antisense gene were 9.8% and 12.7%, respectively (Tang et al., 1994, Chinese Science Bulletin, 39(24): 2280-2283). No doubt, the most successful transformation system is built on the better tissue culture system in the field of plant genetic engineering.
There are lots of studies on tissue culture of tomato; much more regeneration system have been established (Ou et al., 2002; He et al., 2003), but it is obvious that low regeneration frequency, instability, and high proportion of deformity bud are still existing (Liang et al., 2004). Therefore, In this research we attempted to study the factors related to tomato cotyledon regeneration in order to establish a stable regeneration system, and to lay the foundation for the genetic transformation of tomato in future.
1 Results and Analysis
1.1 Screening seed disinfection methods and obtaining aseptic seedlings
The results showed that the seeds treated with mercuric chloride had low seed germination rate, long germinating time and irregular germination, whereas the seeds treated with sodium hypochlorite had higher seed germination rate, short germination time and identical germination. If tween-20 was added into sodium hypochlorite, the effects of sterilization will be better and the germination rate can reach 90% up (Figure 1A). Summing up the above results, treating tomato seed with 70% ethanol for 30 s and then 20% sodium hypochlorite with tween-20 for 20 min would be more appropriate method for seed surface sterilization (Table 1).
 Table 1 Effects of different sterilizing methods on tomato seed surface disinfection |
 Figure 1 Establishment of regeneration system from cotyledon of Chinese tomato |
1.2 The effects of different hormone combinations on tomato bud differentiation
Using MS medium (N & N-organic) as primary medium combining the different hormones, the regeneration frequency of the combinations (Table 2) showed that tomato cotyledon differentiation rate was higher at the level of ZT 2.0 mg/L. Almost every tested explants could differentiate callus tissues and adventitious buds; and the robust combination was ZT 2.0 mg/L and IAA 0.1 mg/L together, which can induce strong buds and less blank buds and deformed buds, and the average budding number reached 5.5 (Figure 1C). The combination of BA and IAA could also induce buds but the number of the induced shoots of each explant was less than that of the combination of ZT and IAA. It is interesting that the combination of BA and NAA is better for the callus growth than other combinations and the callus tissues were 2-fold larger more than others (Figure 1B). However, the callus tissues was obviously green, and tissue structure was swell and loose, but there was no bud forming any more, which indicated that the combination of NAA and BA should facilitate to callus tissue growth but not to bud formation. The results showed that the medium of MS + ZT of 2 mg/L + IAA 0.1 mg/L, had the highest tomato bud induced rate, while the best medium for inducing tomato callus tissues was MS + BA 1.0 mg/L + NAA 1.0 mg/L.
 Table 2 Effects of different hormone combination on induction of adventitious buds |
1.3 The effects of the different types of explants on bud differentiation
Steriled tomato cotyledon, leaf, stem and root segments were placed on the selected best bud inducing medium; after 4 day culture, it could see cotyledons and leaves expanding significantly, thickening, color fading but lighting; while stem segments became thickening, elongating, going to yellowish and a little bit villous aerial root appearing. After 5 days later, the bottom of the root segment and the edges of cotyledon and leaf appeared callus tissues in various degrees. It was found that the color of cotyledon callus had more green and more compact, and the callus tissue from stem segments was looser than that from cotyledon, there were some white clustering sticks occurring at the top of the stem segments. Continuing to culture other 2 or 5 days, the callus edges of the cotyledons, leaves and stems generated adventitious buds, but not for the root segments that were serious browning. In comparison of several explants, the differentiation rate for the cotyledons can reach 83.5%. Stem segments followed by 77.0% (Table 3; Figure 1D).
Table 3 Effects of different kinds of explants on induction of adventitious buds |
1.4 The effects of the different parts of cotyledon on morphogenesis
The whole cotyledon was cut into three segments (tip, middle and end) as different explants that were placed on the same medium for inducing callus. After seven day culturing, it can see the tip explants were beginning to appearing signs of turning yellowish, a few of the middle and the end explants appeared yellowing; while the callus tissues appeared on the tip, middle and end of cotyledons in the different extent. After 10 days later, the outer edge of the explant callus tissues generate the adventitious buds. Statistical analysis showed that the differentiation rates of the middle and the end reached 100%, but the number of induced buds of the middle segment was ar best, followed bu the end segements (Table 4). It had a suggestion that the part of cutting wound in explant would be easy to induce callus and adventitious buds, the middle segment of cotyledons would be the best explant to induce differentiation of tomato buds.
Table 4 Effects of different cotyledon part on induction of adventitious buds |
1.5 The effects of cotyledons placing ways on the bud differentiation
The middle segment of the cotyledon placed on the bud inducing medium by two different ways, face up and back up. The face-up explants were going to be serious yellowish, but the back-up explants grew well after one week later. The edge of callus induced from the back-up explants occured lots of the adventitious buds (Table 5) while the face-up cotyledons induced only a few buds. It is obvious that under the same conditions, the the way of the back-up placing should be more conducive to the induction of adventitious buds.
Table 5 Effects of different way of placement on induction of adventitious buds |
1.6 The adventitious buds rooting
The adventitious buds about 1 to 2 cm in length were transferred to rooting medium for in ducing roots, it was normal to generate roots after 3 to 5 days culturing. After two weeks it can see that the 1/2 MS medium adding the additional of IAA (0.1 mg/L, 0.2 mg/L, 0.3 mg/L) was the best to induce normal milky white and health roots with more lateral roots, the average length of the roots was longer; thereinto, the 1/2MS medium adding IAA 0.1 mg/L is the best for tomato callus rooting resulting in health and thicken roots (Figure 1E). Whereas the 1/2 MS medium adding the additional of NAA (0.1 mg/L, 0.2 mg/L, 0.3 mg/L) for inducing roots was slower to generate roots. In comparison of three treatments, 1/2MS medium adding NAA 0.2 mg/L is better but the root color appeared gray and short and weak roots, not suitable for tomato rooting induction. Definitely, the control medium without adding any hormone can induce the callus rooting, but the induced roots were week, thin and long even long time cultured. It suggested that adding IAA for inducing roots should be more effective than that of adding NAA; and the best rooting medium should be 1/2MS + IAA 0.1 mg/L.
Contamination control is the key factor to success of the trial in plant tissue culture. Therefore, complete disinfection of the explants is the premise of contamination control. According to the different plant species and explant, it should adjust the concentration of disinfectant and treating time accordingly. Seed surface of tomato has tiny villi, incomplete disinfection will result in failure in the whole trial. In this research we thought that the disinfection for tomato seed surface should adopt the procedure as 70% ethanol immersing for 30 s and then rinsing 20% sodium hypochlorite with Tween-20 for 20 min, which was proved to achieve very good disinfection effect.
Plant tissues as explants used for transformation are usually hypocotyl, petiole, cotyledon and stem segments and so on; it is more commonly used for hypocotyl, cotyledon, and petiole. Cotyledons and hypocotyls of tomato are the most employed explants for regeneration in vitro. The study of Sun et al (2006) indicated that two kinds of tomato explants, cotyledons and hypocotyls, could induce callus and adventitious buds, obviously the conclusion of this research is consistent with this.
Plant hormones are indispensable key substance in the medium, which play an important and significant roles in regulating in explant callus induction and root, bud and organ differentiation. In tomato tissue culture, plant hormone, IAA, NAA, 2,4-D, ZT and 6-BA and so on are commonly used to inducing the adventitious buds. Wang et al (2007) had shown that the combinations of ZT and IAA was the best to induce buds of tomato callus than other combinations, which budding under the same conditions at least was as early as 2~5 days, This studies have shown that ZT and IAA combination for inducing buds was the best combination that was good for callus growth.
The explants placed in the different ways would result in differentiation rate of cotyledon bud. This study has shown that back-up placing of the cotyledon is the best way. Regarding the leaves, it is generally believed that the leaf back of dicotyledon plants has lots of stoma, so leaf the back of leaf placing pre-cultured would be conducive to nutrient absorption, therefore, this would be the reason that the explant leaf were commonly exposed to the medium with the leaf back. However, Shi et al (1999) showed that face leaves exposed to the medium had the best effects than that of the back leaves exposed to the medium. Definitely, the placement of leaf explants would affect the induction of adventitious buds but there may also be differences due to different plant species.
Rooting is the key to establish the regeneration system in plant tissue culture; the quality and the rooting rate would determine whether or not that tissue culture technique could be applied in the production. There are many factors affecting the rooting, such as physiological and biochemical status of plantlets itself, primary medium, growth regulators, and other external factors, but plant hormones may be the most important internal regulatory factor. Most of the rooting cases would be achieved with auxin alone (Zhao et al., 2009). However, our experiment suggested that the 1/2MS added IAA was good for inducing roots, indicating that different hormones should be differenced to rooting.
3 Materials and Methods
3.1 Experimental materials and site
This experiment was carried out at tissue culture lab in College of Horticulture of Shanxi Agricultural University; the tested tomato variety named Zhongshu Si Hao was purchased from Shanxi Academy of Agricultural Sciences.
3.2 Treatment of seed disinfection
Tomato seeds were rinsed with fresh water, and then immersed in 70% alcohol for 30 s, transferred to 0.1% mercuric chloride, 20% sodium hypochlorite and 20% sodium hypochlorite with Tween-20. of which treated with 0.1% mercuric chloride for 20 s, 30 s, 1 min, 3 min, respectively; treated with 20% sodium hypochlorite for 10 min, 15 min, 20 min and 30 min, respectively; treated with 20% sodium hypochlorite with Tween-20 same as that without tween-20. The 50 seeds were used for each treatment, repeated three times. Seed germination number and contamination rate were investigated after seed germination.
3.3 Induced bud medium screening
8 days after seed germination, unfolding cotyledons were cut into pieces with the size of 0.5 cm × 0.5 cm to be as explants, some pieces with a petiole and or some pieces from the middle of the leaves. The media for adventitious bud differentiation are the MS (N & N organic) medium adding the different combinations of BA and IAA, BA and NAA, ZT and IAA, with different concentrations. 5 bottles for each combination, four explants placed in a bottle, repeated three times. The induced callus rate and induced bud rate were investigated after 20 days culture.
3.4 The effects of different explants on bud differentiation
Steriled tomato cotyledon, leaf, stem and root segments were placed on the selected best bud inducing medium Vaccine of true leaves, cotyledons, stems and roots (size: 5 mm×5 mm), 20 pieces per explant were placed in the four bottles, repeated three times. The type with highest rate of induced bud was chosen.
3.5 The effects of the different parts of cotyledon on morphogenesis
Employing the selected optimum medium described by 3.3 section, the cotyledons cut into three parts (Figure 2), 20 cuttings of each par were flat placed on the differentiation medium, repeated by three times, the part with highest rate of the bud differentiation was determined.
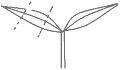 Figure 2 The cotyledon was cut into three parts |
3.6 The effects of cotyledons placing ways on the bud differentiation
Employing the selected optimum medium described by 3.3 section, cotyledon was placed in the medium in two ways, face up and back up, each trial sets five bottles, four explants per bottle, repeated 3 times, callus inducing rate and budding inducing rate were investigated after 20 days culture.
3.7 The adventitious buds rooting
when the adventitious shoots grow up to about 2 cm, the bud at the base was cut to insert in the rooting medium, the culture medium was 1/2 MS medium supplemented with different concentrations of IAA (0, 0.1 mg/L, 0.3 mg/L, 0.5 mg/L) and different concentration of NAA (0, 0.1 mg/L, 0.3 mg/L, and 0.5 mg/L). After two weeks we start to determine the best medium for rooting by the observation and comparison.
3.8 Light conditions
Photoperiod conditions for tissue culture were set as 16 h/8 h (light/dark). Culture shelves for light culture with illumination intensity was from 2 000 to 3 000 Lx and temperature at (25±1)?; Biochemical incubator for dark culture with the temperature at (25±1)?.
Acknowledgement
This research was co-supported by Shanxi Province Returned Overseas Students Scientific Research Projects (2007062), Shanxi Province Agriculture and Social Development Science and Technology Projects (20090311022), and Shanxi Province Agricultural Science and Technology Research Projects (20110311015-1).
References
Frary A., and Earle E.D., 1996, An examination of factors affecting the efficiency of Agrobacterium-mediated transformation of tomato, Plant Cell Report, 16(3-4): 235-240
http://dx.doi.org/10.1007/BF01890875 http://dx.doi.org/10.1007/s002990050214
Gao Z.Y., 2006, Research status of allium macrostemon bunge and its exploitation, Anhui Nongye Kexue (Journal of Anhui Agricultural Sciences), 34(9): 1864-1865
He X.X., Lu Y.M., Bai J.Y., and Li Y.F., 2003, The establishment of tissue culture of tomato and the study of influence factor, Neimenggu Minzhu Daxue Xuebao (Journal of Inner Mongolia University for Nationalities), 18(1): 30-33
Huang Y.F., Wang Q.Y., Fu G.R., Zhao X.X., and Yang Z.X., 1997, The research on introducing flounder antigreeze protein gene (afp) into tomato, Shengwu Huaxue Zazhi (Journal of biological chemistry), 13(4): 418-422
Liang M.X., Li J.F., Xie L.B., and You H.B., 2004, Problem and solving method in tissue culture of the tomato, Beifang Yuanyi (Northern Horticulture), 3: 74-75
Ou Y.B., Li H.X., Zhang J.H., and Ye Z.B., 2002, Transgenic tomato regenerated from hypocotyl explants, Huazhong Nongye Daxue Xuebao (Journal of Huazhong Agricultural University), 21(3): 206-209
Pozueta-Romero J., Houlné G., Cañas L., Schantz R., and Chamarro J., 2001, Enhanced regeneration of tomato and pepper seedling explant s for Agrobacterium-mediated transformation, The Plant Cell, Tissue and Organ Culture, 67(2): 173-180
http://dx.doi.org/10.1023/A:1011997926381
Sanford J.C., Skubik K.A., and Reisch B.I., 1985, Attempted pollen mediated plant transformation employing genomic donor DNA, Thero. Appl. Genet., 69(5-6): 571-574
http://dx.doi.org/10.1007/BF00251106
Shi X.X., Du G.Q., Gao Y., Ma B.K., Xiao J.H., and Jia J.H., 1999, High adventitious shoot regeneration from leaves in vitro of apple cultivars, Guoshu Xuebao (Journal of Fruit Science), 16(4): 255-258
Sun T.H., Sun X.L., Bo P.F., and Du X.H., 2006, Establishment of in tamato regeneration system, Anhui Nongye Kexue (Journal of Anhui Agri. Sci.), 34(24): 6467-6487
Tian C.E., Wang Z.X., Chen T., Zhou Y.P., Huang Z.R., and Huang Y.D., 2000, Transfer of antibacterial peptide D gene into tomato and identification of transgenic plants, Yichuan (Hereditas), 22(2): 86-89
Van Roekel J.S.C., Damm B., Melchers L.S., and Hoekema A., 1993, Factors influencing transformation frequency of tomato (Lycopersicon esculentum), Plant Cell Report, 12(11): 644-647
Wang Q.H., Ge C.H., Cao S.J., Zhang H.C., Li S.M., and Yin G.X., 2007, Regeneration and optimization of transformation system in tomato, Qingdao Nongye Daxue Xuebao (Journal of Qingdao Agricultural University (Natural Science)), (1): 24-27
Ye Z.B., Li H.X., and Zhou G.L., 1994, Genetic transformation of antisense cDNA of polygalacturonase in tomato and transgenic plant regeneration, Yuanyi Xuebao (Acta Horticulturae Sinica), 21(3): 305-306
Zhao H., Hou L.P., Wang H., Zhang X.Z., and Li M.L., 2009, Factors affecting plant regeneration of cotyledons of Chinese cabbage, Fenzi Zhiwu Yuzhong (Molecular Plant Breeding), 7(5): 972-977
. PDF(0KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Wei Zhang
. Leiping Hou
. Hui Zhao
. Meilan Li
Related articles
. Tomato
. Cotyledon
. Hormone
. Bud induction
Tools
. Email to a friend
. Post a comment


