2. Hubei Key Laboratory of Economic Forest Germplasm Improvement and Resource Comprehensive Utilization, Huanggang Normal University, Huanggang, 438000, P.R. China
3. Science and technology training center of Kunming, Kunming, 650021, P.R. China
* These authors contributed equally to this work
 Author
Author  Correspondence author
Correspondence author
Bioscience Methods, 2012, Vol. 3, No. 3 doi: 10.5376/bm.2012.03.0003
Received: 05 Mar., 2012 Accepted: 03 May, 2012 Published: 08 May, 2012
Dong et al., 2012, Callus Induction and Plant Regeneration from Rosemary Leaves, Bioscience Methods, Vol.3, No.3 21-26 (doi: 10.5376/bm.2012.03.0003)
Rosmarinus officinalis L. leaves were used as explants in the study to investigate factors affecting callus induction and plant regeneration. It was found that the culture medium with higher concentration of sucrose promoted callus induction. MS medium supplemented with 6-BA 0.5 mg/L, NAA 0.5 mg/L, and sucrose 50 g/L showed the best result with an induction rate of 88.8%. When regenerated callus develops shoots, it was found that MS medium containing 6-BA 1.5 mg/L, KT 0.5 mg/L and NAA 0.5 mg/L showed relative good result with 50% regeneration rate. When grown in 6-BA 0.8 mg/L and NAA 0.5 mg/L, the amount of growth was increased by more than 300%. MS medium containing NAA 0.1 mg/L showed better result in inducing roots with rooting rate reaching 65%. Additionally, the study also showed that even though the explants were sterile, solution containing 75% ethanol with other sterilizing reagents would result in mass explant deaths.
Introduction
Rosmarinus officinalis L. (Family Lamiaceae), which is known as Rosemary, is a kind of perennial evergreen shrub. It originates in Mediterranean region, and has been cultivated as a common household plant around the world for long time (Zhang et al., 2006; Minaiyan et al., 2011). R. officinalis L. usually has three basic function as raw materials. Firstly, it is used as spice products in food and beverage, secondly, the essential oil can be added into cosmetic production, lastly, in terms of numerous antioxidants, the plant or the essential oil is the materials of gastrointestinal ailments, which has great effect in various spasmodic conditions such as renal and biliary colic. So far, numerous pharmacological studies have suggested Rosmarinus officinalis L. may have a high therapeutic potential in inflammatory bowel diseases (Minaiyan et al., 2011).
Nowadays, the nature extracts efficacy is a hot spot research. For example, Rosa et al (2011) have utilized ethanol extract from R. officinalis L to investigate that the spasmolytic activity has relationship with calcium channels switch in guinea pig ileum. Omri et al (2011) have tested the expression of nucleoside diphosphate kinase (NDPK) and heat shock protein(HSP) was controlled by luteolin, carnosic acid, and rosmarinic acid. Interestingly, essential oils from R. officinalis L. are likely to kill mites (Martinez-Velazquez et al., 2011), and antioxidant and antigenotoxic effects of its extracts are observed in Salmonella typhimurium TA98 and HepG2 cells (Zegura et al., 2011).
In food industrial, essential oils is coming from natural plant, so, are often used as spices and anticorrosive additives. The compounds of R. officinalis L. (such as Flavonoid, Phenolic, Piperitone, alpha-pinene, Limoene and 1,8 -Cineole from ) are various due to the plant species and the harvest time, especially in the flowering stage (Papageorgiou et al., 2008; Rasooli et al., 2008). Gómez-Estaca et al (2010) have done an antimicrobial research about that the effect of essential oil from R. officinalis L. on 18 genera of bacteria, the result showed that it had antibacterial function. Rasooli et al (2008) have demonstrated that the natural products of R. officinalis L. could inhibited the aflatoins which were produced by aspergillus flavus and harmful to human beings and livestock. When extracts and vitamin C were used together, it appeared stronger oxidation resistance (Djenane et al., 2003). Furthermore, R. officinalis L. can be used for soil and water conservation, city landscaping and family potted (as mozzie buster). Therefore, it’s a kind of economic plant that has prosperous exploiting and utilizing value (Li et al., 2006).
With the increasing demand of R. officinalis L. on food processingã€medical, beauty and health care, this not only enhances the R. officinalis L processing industry, but also the rise of effective and high quality cultivation, therefore, it requires higher quality breeding and cultivation of rosemary.
Usually, there are three propagation methods: seed breeding, and cutting propagation. However, seed germination rate is quite low (about 13%) that it’s not easy to survival by cutting propagation. Although laying propagation has high survival rate, there are no enough plantlets for large area cultivating (Chen and Kang, 2009).
Despite of controversial breeding, some significant biotechnologies which are beneficial to improvement of economic production and food crops, such as genetic engineering, cell engineering, haploid induction and somatic mutation, largely depended on the techniques that explants can regenerate and become intact plants (Vijendra et al., 2005). We adapt in vitro culture techniques, select the rosemary as the explants in this research, induces the leaves to dedifferentiation and become callus, later becomes adventitious buds and finally grow to intact plant. This not only offers the callus and intact plants to rosemary improvement by modern biotechnologies, but also quickly obtain large amount of rosemary materials that the genetic traits are similar for large-scale cultivation. Meanwhile, it establishes the rosemary callus cultivation system in order to produce secondary metabolism product by cell engineering techniques.
1 Results and Analysis
1.1 Screening the optimal sterilization reagents
The treatment of different sterilizing reagents showed that 0.2% HgCl2 (w/v, 5 min) was the optimal treatment (Table 1). From the table 1, we knew that, with 75% (v/v) ethanol, the contamination rate of explants was low as well as the survival rate and sometimes all of them were dead. It was estimated that when the alcohol-soluble substances of R. officinalis L may be dissolved, thus, the leaves were dead. Therefore, the explants sterilization cannot use 75% (v/v) ethanol, which will result in large amount of explants dead.
Table 1 Effect of different sterilizing reagents on Rosmarinus officinalis L. explants |
1.2 Callus induction of R. officinalis L. leaves
The mix proportion test of different concentration of sucrose and hormone indicated that MS medium with 6-BA 0.5 mg/L, NAA 0.5 mg/L, and 50 g/L sucrose was proved to be the optimal medium for the production of calli, which was able to start to dedifferentiation after 6 days. There are more explants that was successful to form callus (88%). However, it needed 9 days that the MS 30 g/L sucrose medium with start to dedifferentiation. From the table 2, we knew that the speed of callus dedifferentiation was much faster in 50 g/L sucrose medium than that in 30 g/L sucrose medium and the growth status of callus was better (Table 2; Figure 1).
Table 2 Effects of mediums with different concentrations of sucrose for callus induction |
 Figure 1 Callus induced from R. officinalis L. leaf explants |
1.3 Regeneration of R. officinalis L. callus
After the dedifferentiation, the leaves became callus, some callus were used to subculture for proliferation subculture, the other callus removed to dedifferentiation medium. The adjustment of cytokinins and auxin ratio in medium was able to dedifferentiate to adventitious buds. The results demonstrated that the MS medium with 6-BA 1.5 mg/L, KT 0.5 mg/L and NAA 0.5 mg/L was proved to be the optimal medium with 50 % regeneration rate, and the number of adventitious buds ranks first place (Table 3).
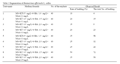 Table 3 Regeneration of Rosmarinus officinalis L. callus |
1.4 Propagating of adventitious shoots
In order to reduce the teratogenic effect on the tube seedlings by the plant hormones accumulation, it was beneficial to reduce the cytokinins and auxin concentration (Table 4). Results indicated that MS medium with 6-BA 0.8 mg/L and NAA 0.5 mg/L was proved to be the best medium and the efficiency can reach 356% (Figure 2).
 Table 4 Propagation for adventitious shoots |
 Figure 2 Adventitious buds propagating |
1.5 Rooting induction for R. officinalis L. shoots
When obtaining enough adventitious buds, it was suitable to reduce the plant growth regulators concentration, which will induce the shoot to a complete plant (Table 5). Among these mediums, the result of the 0.1mg/L NAA MS medium with the 65% rooting rate was better. However, the length of adventitious buds needed to be longer than 1.5 cm in order to obtain better root, whilst the length of adventitious buds was shorter than 1.5 cm, the roots grew fewer and slower, so did the whole plantlet (Figure 3).
Table 5 Rooting induction for Rosmarinus officinalis L. shoots |
 Figure 3 Effect of different bud’s length on rooting |
2 Discussion
With the increasing of people living standard, natural extracts and essential oils of R. officinalis L. were widely used in terms of their aromatic, antiseptic antioxidant effects. Thus, the requirement of rosemary cultivation and breeding was predicted to increase day by day. Either the controversial methods or modern biotechnologies, such as genetic engineering, cell engineering, haploid induction and somatic mutation, was likely to obtain the good quality rosemary, but biotechnological approaches in breeding and cultivation largely depended on an efficient regeneration of intact plants through in vitro systems (Vijendra et al., 2005). This research utilized the leaves as explants, through the Plant in vitro culture techniques, induced the leaves to callus and dedifferentiate to adventitious buds, which will become intact plants. This not only applied good receptor which makes use of enhancing the rosemary quality by modern biotechnologies, but also quickly produced large amount of plants with similar genetic traits. This became the theoretical basis for rosemary in vitro experiment. Simultaneously, this also provided the theoretical basis that extract the effective ingredient from callus directly.
The results also showed that 75% ethanol (30~40 s) with other sterilizing reagents could result in large explants death, which suggested that there were abundant ethanol-soluble components in R. officinalis L. leaf. If utilizing ethanol sterilizing reagents, the content will be dissolved and the cell die. Therefore, the 75% ethanol mixed sterilizing reagents is not suitable for this research. Meanwhile, MS medium with higher sucrose concentration (50 g/L) will promote rosemary leaves to dedifferentiation. This suggested that the rosemary leaves contain lots of essential oil, higher carbon source ratio that is beneficial to enhance the growth of rosemary with Alcohol-soluble substances and lipid. All of results provided the theory for the plant in vitro culture.
3 Materials and Methods
3.1 Materials
Leaf explants from spring shoots of R. officinalis L.; Basal medium of Murashige and Skoog (MS) (Murashige and Skoog, 1962) with 3%~5% (w/v) sucrose, 0.7% Agar, pH 5.8 and different concentration of plant growth regulators;. sterilizing these mediums under 121℃, 0.1-0.5kPa 20 min; distilled water; 75% (v/v) ethanol; 0.2% HgCl2 (w/v); 2% sodium hypochlorite (NaClO, v/v).
3.2 Comparative study of the sterilization effects of the explants primary cultivation
R. officinalis L. leaves and spring stems (3~5 cm) bought from market, washed by running water for 0.5~1 hours, sterilized by different sterilizing reagents (Table 1), washed by sterile still water for 3~5 times, and cultured. The temperature is (25±2)℃ with 70% ~ 80% relative humidity. The light intensity is 2 500 Lux with 14 hours in the daytime and 10 hours at night. After cultured 3 d, 6 d, the status of explants were observed and then record the data every 10 days. or without pre-sterilized in 70% (v/v) ethanol for 30~40 s, and then in commercial bleach (2% sodium hypochlorite) for 15 min, or 0.2% HgCl2 (w/v) for 5 min (Table 1), and then washed 3 to 5 times in distilled water. Leaves were excised from young stems and placed on solid agar sterile medium for callus induction. They maintained in the culturing room at (25±2)℃ and in a 14 h light (2 500 Lux), 8 h dark photoperiod.
3.3 Callus induction of R. officinalis
To investigate the callus induction medium, this research designed mixture of 30 g/L source, 50 g/L source with cytokinins and auxin, separately (Table 2). Note: callus induction (%) = No. of calli from leaf / No. of cultured leaf × 100%; Start-up period (unit; d): The days are from the cultivation to 50% leaf blades that began to differentiate and form calli.
3.4 Regenerating of R. officinalis L. calli
When calli expanded to 1.5~2.0 cm2, some of them continued to subculture in the original medium, while the other induce to dediffireciate to roots with different cell division concentration. This research has designed various cytokinins and auxin ratio to investigate the callus dedifferentiation conditions. Note: budding rate (%) =No. of budding calli (piece of) / No. of culturing calli (piece of) × 100%.
3.5 Propagation of R. officinalis L. adventitious buds
Adjusting the auxin concentration in medium, the rosemary adventitious buds is predicted to induce the lateral buds propagation. The auxin compose and densities is in Table 4. Note: Proliferation rate = No. of shoots / No. of transferring shoots×100%.
3.6 The rooting and the formation of complete plants
In the process of plant tissue culture, medium inducing shoots would inhibit the root growth, and vice versa. Therefore, The adventitious buds that coming from callus didn’t have root. It needed to reduce cytokinins and auxin concentration to grow into an intact plantlet. Select the adventitious buds with 2~5 cm in length to rooting and set up three different NAA concentration; When roots came into 3~6 with about 2~4 cm in length, they could transfer into the soil to grow up.
Author Contributions
YMD and ZNL conceived the overall study, performed the experiment designs, and drafted the manuscript. CQ, BGL and RLD took part in some experiments. YTL supervised the projects and revised the whole written-up. All authors have read and approved the final manuscript.
Acknowledgement
This work was supported by open fund of Hubei Key Laboratory of Economic Forest Germplasm Improvement and Resource Comprehensive Utilization (No. : 2011 BLKF244) and Scientific Research Project fund of Yunnan Provincial Educational Department (No. : 2011Y441).
Reference
Chen D.M., and Kang X.P., 2009, Eco-adaptability and reproduction technique of Rosmarinus offcinalis in Qiannan Region of Guizhou, Guizhou Nongye Kexue (Guizhou Agricultural Sciences), 37(5): 25-27
Djenane D., Sánchez-Escalante A., Beltrán J.A., and Roncalés P., 2003, Extension of the shelf life of beef steaks packaged in a modified atmosphere by treatment with rosemary and displayed under UV-free lighting, Meat Science, 64(4): 417-426
http://dx.doi.org/10.1016/S0309-1740(02)00210-3
Gómez-Estaca J., López de Lacey A., López-Caballero M.E., Gómez-Guillén M.C., and Montero P., 2010, Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation, Food Microbiol., 27(7): 889-896
http://dx.doi.org/10.1016/j.fm.2010.05.012 PMid:20688230
Li X.C., Zhang H.T., Zhou L.H., Li X.W., Lin X.P., and Zeng L., 2006, Tissue culture techniques of buds in Rosmarinus officinalis L., Jingjilin Yanjiu (Economic Forest Researches), 24(3): 15-20
Martinez-Velazquez M., Rosario-Cruz R., Castillo-Herrera G., Flores-Fernandez J.M., Alvarez A.H., and Lugo-Cervantes E., 2011, Acaricidal effect of essential oils from Lippia graveolens (Lamiales: Verbenaceae), Rosmarinus officinalis (Lamiales: Lamiaceae), and Allium sativum (Liliales: Liliaceae) against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae), Journal of Medical Entomology, 48(4): 822-827
http://dx.doi.org/10.1603/ME10140 PMid:21845941
Minaiyan M., Ghannadi A.R., Afsharipour M., and Mahzouni P., 2011, Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats, Research in Pharmaceutical Sciences, 6(1): 13-21
PMid:22049274 PMCid:3203268
Murashige T., and Skoog F., 1962, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Physiolgia Plantarum, 15(3): 473-497
http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
Omri A.E.L., Han J., Ben Abdrabbah M., and Isoda H., 2011, Down regulation effect of Rosmarinus officinalis polyphenols on cellular stress proteins in rat pheochromocytoma PC12 cells, Cytotechnology (doi: 10.1007/s10616-011-9352-y)
http://dx.doi.org/10.1007/s10616-011-9352-y PMid:21861121
Papageorgiou V., Mallouchos A., and Komaitis M., 2008, Investigation of the antioxidant behavior of air- and freeze-dried aromatic plant materials in relation to their phenolic content and vegetative cycle, Journal of Agricultural and Food Chemistry 56(14): 5743-5752
http://dx.doi.org/10.1021/jf8009393 PMid:18578534
Rasooli I., Fakoor M.H., Yadegarinia D., Gachkar L., Allameh A., and Rezaei M.B., 2008, Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils, International journal of food microbiology,122(1-2): 135-139
http://dx.doi.org/10.1016/j.ijfoodmicro.2007.11.048 PMid:18190993
Ventura-Martínez R., Rivero-Osorno O., Gómez C., and González-Trujano M.E., 2011, Spasmolytic activity of Rosmarinus officinalis L. involves calcium channels in the guinea pig ileum, Journal of Ethnopharmacology, 137(3): 1528-1532
http://dx.doi.org/10.1016/j.jep.2011.08.047 PMid:21896322
Vijendra K. Sharma, Robert Ha¨nsch, Ralf R. Mendel, and Jutta Schulze, 2005, Jutta schulze, mature embryo axis-based high frequency somatic embryogenesis and plant regeneration from multiple cultivars of barley (Hordeum vulgare L.), Journal of Experimental Botany, 56(417): 1913-1922
http://dx.doi.org/10.1093/jxb/eri186 PMid:15911560
Zegura B., Dobnik D., Niderl M.H., and FilipiÄ M., 2011, Antioxidant and antigenotoxic effects of rosemary (Rosmarinus officinalis L.) extracts in salmonella typhimurium TA98 and HepG2 cells, Environ Toxicol Pharmacol., 32(2): 296-305
http://dx.doi.org/10.1016/j.etap.2011.06.002 PMid:21843811
Zhang S.H., Weng J.Z., Lin J.B., Chen Y.K., and Lin J.G., 2006, Techniques for tissue culture and rapid propagation of rosemary (Rosemarinus officinalis L.), Guangxi nongye kexue (Guangxi Agricultural Sciences), 37(2): 111-112
. PDF(549KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Yumei Dong
. Renxiang Wang
. Zhengnan Li
. Cheng Qi
. Baogang Liu
. Rulan Duan
. Yating Liu
Related articles
. Rosmarinus officinalis L.
. Leaf explant
. Callus
. Differentiation
. Regeneration
Tools
. Email to a friend
. Post a comment


