Research Article
Cloning and Bioinformatics Analysis of Genes Related to Key Enzymes in Oil Metabolism of Carya illinoensis (Wangech.) K. Koch 
2 The Jiangsu Provincial Platform for Conservation and Utilization of Agricultural Germplasm, Nanjing, 210014, China
 Author
Author  Correspondence author
Correspondence author
Bioscience Methods, 2022, Vol. 13, No. 1 doi: 10.5376/bm.2022.13.0001
Received: 25 Nov., 2021 Accepted: 07 Jan., 2022 Published: 18 Jan., 2022
Jia Z.H., Jia X.D., Xu M.Y., Mo Z.H., Yang X.F., Zhai M., Xuan J.P., Zhang J.Y., Wang G., Wang T., and Guo Z.R., 2022, Cloning and bioinformatics analysis of genes related to key enzymes in oil metabolism of Carya illinoensis (Wangech.) K. Koch, Bioscience Method, 13(1): 1-13 (doi: 10.5376/bm.2022.13.0001)
Taking mixed sample of 115 d and 135 d Carya illinoinensis ‘Pawnee’ as experimental materials, after RT-PCR amplification, cloning and sequencing, the gene sequences of CiSAD gene, CiFAD2 gene and CiGPAT gene related to the key enzymes of oil metabolism in Carya illinoinensis were obtained, and bioinformatics analysis was carried out. The length of CiSAD gene was 1 240 bp, containing a complete open reading frame (ORF) of 1 194 bp, encoding 397 amino acids. CiFAD2 was 1 329 bp, containing an ORF of 1 155 bp, encoding 384 amino acids. CiGPAT was 1 671 bp, containing an ORF of 1 626 bp, encoding 541 amino acids. All proteins encoded by the cloned genes have corresponding domains, indicating that the proteins encoded by these genes should have corresponding functions in organisms.
Pecan (Carya illinoensis (Wangech.) K. Koch) originated in the United States. At present, the pecan central producing area is the United States, and distributed in the Mexico, Italy, France, Israel, Japan, China and other places (Zhang and Lv, 1998, Guangxi Forestry Science, 27(4): 202 - 206). Carya illinoensis can be traced back to the Cretaceous, and it is one of the important dry fruit trees in the world (Wang et al., 2009; 2010). Compared with Juglans regia and Carya cathayensis in China, Carya illinoensis has thin shell with high yield (1 500~2 250 kg/ha) and high oil yield, which is easy to extract kernel. And Carya illinoensis contains higher unsaturated fatty acids (UFA) and various amino acids beneficial to human body than olive, but also rich in vitamin B1, B2 and vitamin E (Pan, 2008, Xiandai Horticulture (2): 12-13; Hong, 2007). The highest content of nutrients in Carya illinoensis kernels is oil. Mature kernels contain 55%~75% oil, and are mainly triglycerides (TAG) composed of glycerol and fatty acids.
The oil content of Carya illinoensis is high. Among them, oleic acid accounts for a high proportion, which is an excellent material for studying the regulation mechanism of lipid metabolism, especially the regulation mechanism of monounsaturated fatty acid (MUFA) metabolism. Stearoyl-ACP desaturase (SAD) catalyzes the dehydrogenation of palmitic acid to produce a double bond to form oleic acid, which affects the proportion of saturated fatty acid (SFA) and MUFA and is the key enzyme in the lipid metabolism pathway of Carya illinoensis. At present, SAD gene has been isolated from various plants such as Arabidopsis thaliana (Ping et al., 2008), Glycine max (Byfield et al., 2006), Arachis hypogaea L. (Florin et al., 2010; Dong et al., 2012), Elaeis guineensis (Shah et al., 2000), Camellia oleifera (Zhang et al., 2008; Zhang et al., 2008) Brassica campestris (Knutzon et al., 1992), Solanum tuberosum (Taylor et al., 1992; Li et al., 2015), Xanthoceras sorbifolia (Zhao et al., 2015). Omega-6 fatty acid desaturase (FAD2) catalyzes the dehydrogenation of oleic acid to produce the second double bond to form linoleic acid, which is another key enzyme in the lipid metabolism pathway of Carya illinoensis. At present, FAD2 gene has been isolated from various plants such as Xanthoceras sorbifolium (Zhao et al., 2015), Brassica napus (Xiong et al., 2002), Glycine max (Li et al., 2007), Olea europaea var sylvestris (Georgios et al., 2005), Gossypium hirsutum (Kargiotidou et al., 2008). Glycerol-3-phosphate acyltransferase (GPAT) catalyzes the first-step of acylation reaction of various glyceride such as triacylglycerol and phosphatidylglycerol, and can acylate the sn-1 position of triacylglycerol (Shockey et al., 2016), which plays a very important role in the accumulation of vegetable oil. In Carya illinoensis, GPAT catalyzes the first step of the TAG synthesis reaction to produce 1-acyl glycerol ester. There are three types of GPAT in plant cells, which are located on plastids, cytoplasm and mitochondria, respectively. GPAT genes have been cloned from Arabidopsis thaliana and other plants (Nishida et al., 1993).
In the transcriptome study of Carya illinoensis kernels during fruit quality formation, we found that the unigenes encoding SAD, FAD2and GPAT genes in the lipid metabolism pathway of Carya illinoensis were highly expressed, indicating that they may play an important role in the lipid metabolism of Carya illinoensis (Jia at al., 2018). In this study, the gene sequences of these three key enzyme genes SAD, FAD2 and GPAT were cloned and analyzed by bioinformatics, to lay the foundation for the in-depth study of high oil mechanism and breeding of Carya illinoensis.
1 Materials and Methods
1.1 Materials
1.1.1 Plant materials
The test material was Carya illinoinensis ‘Pawnee’, which was cultivated in the Production Base of Nanjing Luzhou Pegan Co., Ltd., Luhe District, Nanjing. The tree-age was 8~12 a, with the spacing in the rows and spacing between rows of 5.0 m×7.0 m. 6 plants with good growth and relatively consistent tree potential were selected, and 2 plants were divided into one plot, which were repeated 3 times. In 2014, samples were taken at 115 d and 135 d after anthesis, and 2 healthy and full fruits without pests and diseases were taken from each plant in four directions of east, south, west and north. Samples were put into the ice box and taken back to the lab quickly. The samples were stored overnight in a refrigerator at -20℃, and then cut open to take kernels. The kernels were frozen and mixed in liquid nitrogen and stored in the refrigerator at -80℃.
1.1.2 Reagent
RNAsimple Total RNA Kit (TIANGEN BIOTECH), HiScript® Ⅱ Q RT SuperMix, (Vazyme), 2 × Taq Master Mix (Vazyme). The primers were synthesized by Shanghai Invitrogen Biotechnology Co., Ltd.
1.1.3 Instrument
SimpliAmp™ PCR, Hettich centrifuge (Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany), pipette (Eppendorf, Germany).
1.2 Methods
1.2.1 Total RNA extraction
Taking mixed sample of 115 d and 135 d Carya illinoinensis ‘Pawnee’ as experimental materials. Total RNA was extracted according to the instructions of RNAsimple Total RNA Kit of Tiangen Biotech (Beijing) Co., Ltd. The ratio of OD260/OD280 of total RNA was determined by ultraviolet spectrophotometer to determine the purity and concentration of RNA. Detected the integrity of RNA with 1% agarose gel electrophoresis.
1.2.2 Reverse transcription
The first strand of cDNA was synthesized according to the HiScript® II Q RT SuperMix for qPCR (+gDNA wiper) instructions as follows: The template RNA, RNase free ddH2O and 4 × gDNA wiper Mix were added to the RNase-free centrifuge tube, and gently mixed with a pipette. The genomic DNA was removed at 42°C for 2 min. Then added 5 × HiScript II qRT SuperMix II to prepare a reverse transcription reaction system, gently mixed with a pipette. Reverse transcription was performed at 50°C for 15 min and 85°C for 5 sec in turn.
1.2.3 Primer design
Primers (Table 1) were designed with the help of the unigene sequence obtained from the previous transcriptome (Jia et al., 2018).
|
Table 1 Sequence of primers for pecan |
1.2.4 PCR amplification of target gene fragment
Refer to the instructions of 2 × Taq Master Mix (Vazyme), and the reaction system was as follows (Table 2).
|
Table 2 Reaction of PCR amplification |
1.2.5 Recovery, ligation, transformation, identification and sequencing of the target fragment
The target fragment was recovered and purified by Biospin agarose gel DNA recovery kit, and the steps were completely in accordance with the instructions. After the recovery, 3 μL of recovered and purified DNA products were taken for 1% agarose gel electrophoresis detection, and the quality of recovered DNA products was detected by ultraviolet spectrophotometer. The recovered DNA solution can be immediately used for ligation reaction or stored at -20°C.
The successfully recovered target fragment was ligated to pMD18-T vector (TaKaRa, Japan), and the reaction system was shown in Table 3. After mixing, reacted at 16°C for more than 4 h or overnight. Then transformed into Escherichia coli and performed colony PCR. The PCR product length of the appropriate monoclonal sent to Genepioneer Biotechnologies for sequencing.
|
Table 3 Reaction of target fragment |
1.2.6 Sequence analysis
The nucleotide sequences obtained by sequencing were compared by BLASTn in NCBI, and then the deduced amino acid sequences were compared by BLASTp. The plant amino acid sequences with high homology were selected for homology sequence alignment and phylogenetic tree construction. Physicochemical property of the protein was analyzed by Swiss-Prot online tool. The isoelectric point, GRAVY value prediction website is https://web.expasy.org/compute_pi/. Protein hydrophobicity/hydrophilicity prediction website is https://web.expasy.org/protscale/. DNAman 7.0 software was used for homologous sequence alignment. CDD (Conserved Domain Database) in CNBI was used for prediction of conservative domains. MEGA 5.1 software was used to construct phylogenetic tree.
2 Results and Analysis
2.1 Cloning and sequence analysis of CiSAD gene from Carya illinoinensis
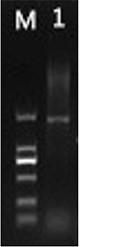
A target gene fragment with a length of about 1 300 bp (Figure 1) was obtained by PCR amplification. After recovery and sequencing, it was found that the sequence length was 1 240 bp. After NCBI alignment, it contained a complete open reading frame (ORF) of 1 194 bp, encoding 397 amino acids, which was named CiSAD. The accession number of this sequence in the GenBank database was BankIt2129876 Seq1 MH588443.
|
Figure 1 Pecan CiSAD and CiFAD2 gene fragment amplified by PCR |
The physicochemical property of the protein was predicted by Swiss-Prot online tool. Results showed that the molecular weight of the protein was 44.99 kDa and the isoelectric point (pI) was 6.77, the grand average of hydropathicity (GRAVY) value was -0.351, indicating that the protein is a hydrophilic protein.
The alignment analysis results of amino acid sequence in CiSAD gene from Carya illinoinensis and other species showed that the CiSADgene from Carya illinoinensis had high homology with oil plants such as Juglans regia, Jatropha curca, Ricinus communis and so on. Especially Juglans regia with the highest homology of 93%, and the homology with other plants was also greater than 80%. The CiSAD amino acid sequence deduced from Carya illinoinensis was subjected to multiple alignment with SAD amino acid sequences with high homology such as Juglans regia, Olea europaea var sylvestris, Manihot esculenta, Jatropha curcas, Ricinus communis, Hevea brasiliensis, Citrus sinensis, Populus trichocarpa and Arabidopsis thaliana. The results are shown in Figure 2. By comparing the SAD amino acid sequences of different species, combined with the CDD database of NCBI, it was found that the SAD protein contains two conserved domains: an acyl-ACP desaturase conserved domain with 256 amino acids from 65 to 320 and a ferritin-like family conserved domain with 129 amino acids from 144 to 272 (Figure 3). These two conserved regions are shared by acetyl-ACP desaturase and are highly conserved in plants.
|
Figure 2 Multiple sequence alignment of amino acid homology of CiSAD Note: CiSAD, Carya illinoensis; JrSAD, Juglans regia (xp_018849207.1); OeSAD, Olea europaea var sylvestris (XP_022879085.1); MeSAD, Manihot esculenta(XP_021603146.1); JcSAD, Jatropha curcas (XP_012066083.1); RcSAD, Ricinus communis (NP_001310674.1); HbSAD, Hevea brasiliensis (XP_010999822.1); CsSAD, Citrus sinensis (XP_006472924.1); PtSAD, Populus trichocarpa (XP_002307478.2); AtSAD, Arabidopsis thaliana (NP_175048.1). Red and green underlines represent the conserved domain of acyl-ACP desaturase family and ferritin-like family |
|
Figure 3 Phylogenetic tree constructed using CiSAD of pecan Note: HbSAD, Hevea brasiliensis (XP_010999822.1); MeSAD, Manihot esculenta (XP_021603146.1); RcSAD, Ricinus communis (NP_001310674.1); JcSAD, Jatropha curcas (XP_012066083.1); PtSAD, Populus trichocarpa (XP_002307478.2); CsSAD, Citrus sinensis (XP_006472924.1); CiSAD, Carya illinoensis; JrSAD, Juglans regia (xp_018849207.1); OeSAD, Olea europaea var sylvestris (XP_022879085.1); AtSAD, Arabidopsis thaliana (NP_175048.1); BnSAD, Brassica napus (CAA65990.1); HaSAD, Helianthus annuus (CAC80359.1); AhSAD, Arachis hypogaea (AAD48495.1); GmSAD, Glycine max (AAA92462.1) |
The phylogenetic tree of Carya illinoensis, high-homologous plants and common oil crops was constructed with MEGA 5, and it was mainly divided into three branches. The first branch consisting of woody oil crops such as Hevea brasiliensis and Manihot esculenta and containing Carya illinoensis, the second branch consisting of Arabidopsis thaliana and the third branch consisting of herbaceous oil crops such as Brassica napus and Helianthus annuus (Figure 3).
2.2 Cloning and sequence analysis of CiFAD2 gene from Carya illinoinensis
The amplification of the CiFAD2 sequence of Carya illinoinensis is shown in Figure 1. The length of the target gene is 1 329 bp, containing an ORF of 1 155 bp and encoding 384 amino acids. The molecular weight of the deduced protein is 44.30 kDa, and the isoelectric point (pI) is 8.79. The grand average of hydropathicity (GRAVY) value is -0.063, indicating that the protein is a hydrophilic protein. The accession number of this sequence in GenBank database is BankIt2133004 Seq1MH613768.
The amino acid sequence of CiFAD2 from Carya illinoensis was aligned with BLASTp in NCBI, and it was found that CiFAD2 had the highest homology with Juglans regia (97%). And the homology with Xanthoceras sorbifolium, Corylus avellana, Quercus suber, Rhus chinensis, Citrus sinensis, Vitis vinifera, Sesamum indicum, Coffea canephora are also more than 80%. The CiFAD2 amino acid sequence of Carya illinoensis was compared with the FAD2 amino acid sequence of plants with high homology. The results are shown in Figure 4. By searching CDD database in NCBI combined with literature reports, it was found that the protein contains 3 histidine conserved domains, namely sequence HLLPH, HECGH and HVAHH (HXXXH or HXXHH). The conserved domain is a putative di-iron ligand shared by the membrane-FADS-like superfamily, which is the binding site of iron ions, and the active center of FAD enzyme catalysis (Shanklin and Cahoon, 1998). The phylogenetic tree was constructed using MEGA 5 (Figure 5). It was found that Carya illinoensis was first combined with Juglans regia, and then combined with Corylus avellana. While herbal oil crops such as Glycine maxand Sesamum indicum had a far evolutionary relationship with Carya illinoensis.
|
Figure 4 Multiple sequence alignment of amino acid homology of CiFAD2 Note: CiFAD2, Carya illinoensis; JrFAD2, Juglans regia (XP_018834629.1); XsFAD2, Xanthoceras sorbifolium (AGO32050.1); QsFAD2, Quercus suber(XP_023886251.1); RcFAD2, Rhus chinensis (AIC34705.1); CcFAD2, Coffea canephora (CDP17521.1); VvFAD2, Vitis vinifera (XP_002285640.1); CaFAD2, Corylus avellana (AIT96965.1); SiFAD2, Sesamum indicum (XP_011080227.1); CsFAD2, Citrus sinensis (XP_006492661.1). *, represent the conserved domain of histidines |
|
Figure 5 Phylogenetic tree constructed using CiFAD2 gene of pecan Note: CiFAD2, Carya illinoensis; JrFAD2, Juglans regia (XP_018834629.1); CaFAD2, Corylus avellana (AIT96965.1); QsFAD2, Quercus suber(XP_023886251.1); PgFAD2, Punica granatum (AAO37754.1); XsFAD2, Xanthoceras sorbifolium (AGO32050.1); RcFAD2, Rhus chinensis (AIC34705.1); CsFAD2, Citrus sinensis (XP_006492661.1); VvFAD2, Vitis vinifera (XP_002285640.1); HbFAD2, Hevea brasiliensis (AAY87459.1); GmFAD2, Glycine max(NP_001347010.1); SiFAD2, Sesamum indicum (XP_011080227.1); CcFAD2, Coffea canephora (CDP17521.1); OeFAD2, Olea europaea var sylvestris(XP_022875273.1) |
2.3 Cloning and sequence analysis of CiGPAT gene from Carya illinoinensis
The target sequence was obtained by PCR amplification (Figure 6). The length of CiGPAT sequence from Carya illinoinensis is 1 671 bp. After NCBI alignment, it contained an ORF of 1 626 bp, encoding 541 amino acids. The molecular weight of the derived protein is 61.25 kDa, isoelectric point (pI) is 8.97, and the GRAVY value is 0.159, indicating that the protein is a hydrophobic protein. The accession number of this sequence in GenBank database is BankIt2133004 Seq2 MH613769.
|
Figure 6 Pecan CiGPAT gene fragment amplified by PCR |
The amino acid sequence of CiGPAT from Carya illinoensis was aligned with BLASTp, and it was found that CiGPAT had the highest homology with Juglans regia (94%). And the homology with Quercus suber, Vitis vinifera and other plants are between 62%~71%. The CiGPAT amino acid sequence of Carya illinoensis was compared with the GPAT amino acid sequence of plants with high homology. The results are shown in Figure 7. By searching CDD database in NCBI combined with literature reports, it was found that the C-terminal of the protein contained Lysophospholipid acyltransferase (LPLAT), PlsC, Acyltransferase, AGP_acyltrn, PLN02177 and other domains. Among them, LPLAT is the functional domain of acyltransferase involved in TAG biosynthesis, Acyltransferase is the functional domain of acyltransferase involved in phospholipid synthesis, and Plsc is the functional domain of glycerol triphosphate acyltransferase involved in lipid synthesis (Heath and Rock, 1998; Nagiec et al., 1993). Using MEGA 5 to construct a phylogenetic tree, it was found that Carya illinoensis and Juglans regia first clustered together, then clustered with Quercus suber, Populus trichocarpa and Populus euphraticaclustered together, and the farthest relationship was Ziziphus jujube and Glycine max (Figure 8).
|
Figure 7 Phylogenetic tree constructed using CiGPAT of pecan Note: CiGPAT, Carya illinoensis; JrGPAT, Juglans regia (xp_018849207.1); QsGPAT, Quercus suber (XP_023927330.1); DzGPAT, Durio zibethinus(XP_022769787.1); RcGPAT, Ricinus communis (XP_015572336.1); HbGPAT, Hevea brasiliensis (XP_021644822.1); PtGPAT, Populus trichocarpa(PNT50557.1); JcGPAT, Jatropha curcas (XP_012082935.1); PeGPAT, Populus euphratica (XP_011002733.1); MeGPAT, Manihot esculenta (XP_021614154.1) |
|
Figure 8 Phylogenetic tree constructed using CiGPAT of pecan Note: HbGPAT, Hevea brasiliensis (XP_021644822.1); MeGPAT, Manihot esculenta (XP_021614154.1); JcGPAT, Jatropha curcas (XP_012082935.1); RcGPAT, Ricinus communis (XP_015572336.1); PtGPAT, Populus trichocarpa (PNT50557.1); PeGPAT, Populus euphratica (XP_011002733.1); CcGPAT, Corchorus capsularis (OMP11935.1); DzGPAT, Durio zibethinus (XP_022769787.1); TcGPAT, Theobroma cacao (EOX95331.1); VvGPAT, Vitis vinifera(XP_002271508.1); QsGPAT, Quercus suber (XP_023927330.1); CiGPAT, Carya illinoensis; JrGPAT, Juglans regia (xp_018849207.1); ZjGPAT, Ziziphus jujube(XP_015891692.1); GmGPAT, Glycine max (AAA92462.1) |
3 Discussion
SAD gene plays an important role in the process of plant oil accumulation, so there are relatively many studies on SAD gene. SAD gene has been cloned from a variety of plants. The coding region of most SAD genes is 1 170~1 190 bp, encoding precursor proteins containing about 390 amino acid residues (Knutzon et al., 1992; Taylor et al., 1992; Shah et al., 2000; Byfield et al., 2006; Ping et al., 2008; Zhang et al., 2008; Florin et al., 2010; Dong et al., 2012; Li et al., 2015; Zhao et al., 2015). The CiSAD gene cloned in this study was 1 194 bp in length, encoding 397 amino acids, including initiation codon ATG and termination codon TAA. The CiSAD amino acid sequence cloned in this study has high homology with other plants, especially with the same family plant Juglans regia, indicating that the SAD gene in plants is relatively conservative. Studies have shown that SAD gene mainly exists in plant chloroplast matrix, with tissue expression specificity and the highest expression level in seeds (Shanklin and Somerville, 1991). Inhibiting the expression of SADgene in Brassica napu could increase the content of stearic acid in Brassica seeds (Knutzon et al., 1992). Similarly, silencing of the SADgene in Gossypium could increase stearic acid content by 20% (Liu et al., 2002), while overexpression of SAD gene in Lycopersicon esculentum could increase oleic acid content by 60% (Zaborowska et al., 2002). The study of 508 maize inbred lines showed that the SAD gene had the greatest impact on the proportion of C18:0/C18:1 in seeds. One nonsynonymous single-nucleotide polymorphism in exon 3 and one 5-bp insertion/deletion in the 3′ untranslated region were further shown to contribute to the natural variation in C18:0/C18:1 in maize (Han et al., 2017). In summary, SAD gene plays an important role in the accumulation of UFA in plants and can regulate the ratio of SFA to UFA. In this study, CiSAD gene from Carya illinoinensis was cloned, which laid a foundation for the regulation of oil metabolism in Carya illinoinensis in the future.
FAD2 belongs to desaturase gene family, its content and activity determine the composition and proportion of UFA in oil. At present, high oleic acid lines of almost all common oil crops have been obtained by transgenic or gene mutation techniques (Auld et al., 1992; Jung et al., 2000; Bruner et al., 2001; Schwartzbeck et al., 2001; Buhr et al., 2002; Liu et al., 2002), the acquisition of these lines was achieved by inhibiting the expression of FAD2 gene, which was the most outstanding achievement in the metabolic engineering of oil crops. Desaturase contains three highly conserved histidine-rich regions: His Box I, His Box II and His Box III. Among them, the 3 histidine conserved domains of the omega-6 fatty acid desaturase are HXXH, HXXHH and HXXHH. In this study, the analysis of the functional domain of FAD2 protein showed that the CiFAD2 amino acid sequence contained 3 histidine conserved clusters, namely HLLPH, HECGH and HVAHH, which combined with di-iron (Moche et al., 2003) and constituted the active center catalyzed by desaturase. Studies have shown that the deletion or substitution of His Box I and His Box II may lead to the decrease of enzyme activity, and the deletion or substitution of His Box III may lead to the loss of enzyme activity (Zhang, 2011). Libisch et al. (2000) recombined the amino acids in the HIS I and HIS II regions of the Borago officinalis Δ6-fatty acid desaturase and the HIS III region of the Borago officinalis Δ8-fatty acid desaturase. The results showed that the recombinant enzyme lost the function of catalyzing the dehydrogenation of C18 fatty acids but could catalyze the dehydrogenation of palmitoleic acid (C16:1) and tetradecanoic acid (C I4:1).
Arabidopsis GPAT gene family contains 10 members, namely AtATS and AtGPAT1~9. Among them AtGPAT9 may play a catalytic role in glycerophosphate (Gidda et al., 2009). At the same time, GPAT in other plants also plays an important role in improving seed oil content and oil quality (Chi et al., 2015; Paya-Milans et al., 2016). In this study, the results of homology analysis showed that the GPAT gene has species distinctive. The GPAT gene has a high degree of variation and low homology among different plants, while it is more conservative among plants of the same family and genus. Heath and Rock (1998) found that H and D in Motif HXXXXD structure are important functional sites for acyltransferase activity. Functional domain prediction also showed that CiGPAT not only has LPCAT1-like domain of lysophospholipid acyltransferase (LPLAT), but also has PlsC, Acyltransferase, AGP_acyltrn, PLN02177 domain, indicating that CiGPAT has the functional domain of acyltransferase.
Authors’ contributions
JXD and XJP are the experimental designers of this research. JZH is the executor of this research, completed the data analysis and manuscript writing. XMY, YXF, ZM participated in the design of the study and performed the statistical analysis. MZH, XJP, ZJY, WG, and WT guided data analysis and paper writing. JXD, XJP and GZR conceived of the study, guided the experimental design, data analysis, writing and revision. All authors read and approved the final manuscript.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31901347), Jiangsu Provincial Department of Science and Technology-Special Project of Science and Technology in Northern Jiangsu (SZ-YC2019038), and Independent Scientific Research Project of Institute of Botany, Jiangsu Province and Chinese Academy of Sciences (JSPKLB202055).
Auld D.L., Heikkinen M.K., Erickson D.A., Sernyk J. L., and Romero J. E., 1992, Rapeseed mutants with reduced levels of polyunsaturated fatty acids and increased levels of oleic acid, Crop Science, 32: 657-662
https://doi.org/10.2135/cropsci1992.0011183X003200030016x
Bruner A.C., Jung S., Abbott A.G., and Powell G.L., 2001, The naturally occurring high oleate oil character in some peanut varieties results from reduced oleoyl-PC desaturase activity from mutation of aspartate 150 to asparagine, Crop Science, 41: 522-526
https://doi.org/10.2135/cropsci2001.412522x
Buhr T., Sato S., Ebrahim F., Xing A., Zhou Y., Mathiesen M., Schweiger B., Kinney A., Staswick P., and Clemente T., 2002, Ribozyme termination of RNA transcripts down-regulate seed fatty acid genes in transgenic soybean, Plant Journal, 30: 155-163
https://doi.org/10.1046/j.1365-313X.2002.01283.x
PMid:12000452
Byfield G.E., Xue H., Upchurch R.G., 2006, Two genes from soybean encoding soluble Δ9-stearoyl-ACP desaturase, Crop Science, 46: 840-846
https://doi.org/10.2135/cropsci2005.06-0172
Chi X., Yang Q., Pan L., et al., 2015, Isolation and expression analysis of glycerol-3-phosphate acyltransferasegenes from peanuts ( Arachis hypogaea L. ), GrasasYAceites, 66(3): e09
https://doi.org/10.3989/gya.1190142
Dong J., Wan Y., and Liu F., 2012, Sequence analysis of Δ9-stearoyl-ACP desaturase gene (SAD) in peanut, Acta Agronomica Sinica, 38(7): 1167-1177
https://doi.org/10.3724/SP.J.1006.2012.01167
Florin S., Yael B., Arnon B., Ilan H., and Ran H., 2010, Identification and molecular characterization of homeologous Δ9-stearoyl-acyl carrier protein desaturase3 genes from the allotetraploid peanut (Arachis hypogaea), Plant Molecular Biology Reporter, 29: 232-241
https://doi.org/10.1007/s11105-010-0226-9
Georgios B., Anastassios M., Nikos N., and Polydefkis H., 2005, Spatial and temporal expressions of two distinct oleate desaturase from olive (Olea europaea L.), Plant Science, 168: 457-555
https://doi.org/10.1016/j.plantsci.2004.09.026
Gidda S.K., Shockey J.M., Rothstein S.J., Dyer J.M., and Mullen R.T., 2009, Arabidopsis thaliana GPAT8 and GPAT9 are localized to the ER and possess distinct ER retrieval signals: Functional divergence of the dilysine ER retrieval motif in plant cells, Plant Physiology Biochemistry, 47: 867-879
https://doi.org/10.1016/j.plaphy.2009.05.008
PMid:19539490
Han Y., Xu G., Du H., Hu J., Liu Z., Li H., Li J., and Yang X., 2017, Natural variations in stearoyl-acp desaturase genes affect the conversion of stearic to oleic acid in maize kernel, Theoretical Applied Genetics, 130(1): 151-161
https://doi.org/10.1007/s00122-016-2800-5
PMid:27717956
Heath R.J., and Rock C.O., 1998, A conserved histidine is essential for glycerolipid acyltransferase catalysis, Bacteriol, 180(6): 1425-1430
https://doi.org/10.1128/JB.180.6.1425-1430.1998
PMid:9515909 PMCid:PMC107040
Hong D.D., 2007, AFLP and SSR analysis of pollinated generation between Carya cathayensis and C.illinoinensis, Zhejiang Forestry University, pp.36-39
Jia X., Li M., Luo H., et al., 2018, Transcriptome survey reveals candidate genes involved in lipid metabolism of Carya illinoinensis, Int J Agric Biol, 20(5): 991-1004
Jung S., Swift D., Sengoku E., Patel M., Teulé F., Powell G., Moore K., and Abbott A., 2000, The high oleate trait in the cultivated peanut (Arachis hypogaea L.). I. Isolation and characterization of two genes encoding microsomal oleoyl-PC desaturases, Molecular General Genetics, 263(5): 796-805
https://doi.org/10.1007/s004380000244
PMid:10905347
Kargiotidou A., Deli D., Galanopoulou D., Tsaftaris A., and Farmaki T., 2008, Low temperature and light regulate delta 12 fatty acid desaturases (FAD2) at a transcriptional level in cotton (Gossypium hirsutum), Journal of Experiment Botany, 2008, 59: 2043 – 2056
https://doi.org/10.1093/jxb/ern065
PMid:18453533 PMCid:PMC2413273
Knutzon D.S., Thompson G.A., Radke S.E., Johnson W.B., Knauf V.C., and Kridl J.C., 1992, Modification of a Brassica seed oil by antisense expression of a stearoyl-acyl carrier protein desaturase gene, Proceeding of the National Academy of Sciences of the United States of America, 89(7): 2624-2628
https://doi.org/10.1073/pnas.89.7.2624
PMid:1557366 PMCid:PMC48714
Li F., Bian C., Xu J., Pang W., Liu J., Duan S., Lei Z., Jiwan P., Jin L., 2015, Cloning and functional characterization of sad genes in potato, PloS One, 10(3): e0122036
https://doi.org/10.1371/journal.pone.0122036
PMid:25825911 PMCid:PMC4380360
Li H.H., Flachowsky H., Fischer T.C., Hanke M., Forkmann G., Treutter D., Schwab W., HoVmann T., and Szankowski I., 2007, Maize Lc transcription factor enhances biosynthesis of anthocyanins, distinct proanthocyanidins and phenylpropanoids in apple (Malus domestica Borkh), Planta, 226: 1243-1254
https://doi.org/10.1007/s00425-007-0573-4
PMid:17618453
Libisch B., Michaelson L.V., Lewis M.J., et al., 2000, Chimeras of delta 6- fatty acid and delta8- sphingolipid desaturases, Biochem Biophys Res Commun
https://doi.org/10.1006/bbrc.2000.4023
PMid:11162428
Liu Q., Singh S.P., and Green A.G., 2002, High-stearic and high-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing, Plant Physiology, 129(4): 1732-1743
https://doi.org/10.1104/pp.001933
PMid:12177486 PMCid:PMC166761
Moche M., Shanklin J., Ghoshal A., and Lindqvist Y., 2003, Azide and acetate complexes plus two iron-depleted crystal structures of the di-iron enzyme deha9 stearoyl-acyl carrier protein desaturase: Im- plications for oxygen activation and catalytic intermediates, Journal of Biological Chemistry, 278(27): 25072-25080
https://doi.org/10.1074/jbc.M301662200
PMid:12704186
Nagiec M.M., Wells G.B., and Lester R.L., 1993, A suppressor gene that enables Saccharomyces cerevisiae to grow without making sphingolipids encodes a protein that resembles an Escherichia coli fatty acyltransferase, The Journal of Biological Chemistry, 268(29): 22156 – 22163
https://doi.org/10.1016/S0021-9258(20)80661-9
Nishida I., Tasaka Y., Shiraishi H., and Murata N., 1993, The gene and the RNA for the precursor to the plastid- located glycerol-3-phosphate acyltransferase of Arabidopsis thaliana, Plant Molecular Biology, 21: 267-277
https://doi.org/10.1007/BF00019943
PMid:7678766
Paya-Milans M., Aznar-Moreno J.A., Balbuena T.S., et al., 2016, Sunflower HaGPAT 9-1 is the predominant GPAT during seed development, Plant Science, 252: 42-52
https://doi.org/10.1016/j.plantsci.2016.07.002
PMid:27717477
Ping Z., Joseph W.B., Robert G.U., Edward W., John S., and Ralph E.D., 2008, Mutations in a Δ9-stearoyl-ACP- desaturase gene are associated with enhanced stearic acid levels in soybean seeds, Crop Science, 48: 2305-2313
https://doi.org/10.2135/cropsci2008.02.0084
Schwartzbeck J.L., Jung S., Abbott A.G., Mosley E., Lewis S., Pries G.L., and Powellg L., 2001, Endoplasmic oleoyl-PC desaturase references the second double bond, Phytochemistry, 57(5): 643-652
https://doi.org/10.1016/S0031-9422(01)00081-4
Shah F.H., Rashid O., and San C.T., 2000, Temporal regulation of two isoforms of cDNA clones encoding delta 9-stearoyl-ACP desaturase from oil palm (Elaies guineensis), Plant Science, 152: 27-33
https://doi.org/10.1016/S0168-9452(99)00209-5
Shanklin J., and Cahoon E.B., 1998, Desaturation and related modifications of fatty acids, Annual Review of Plant Physiology and Plant Molecular Biology, 49: 611-641
https://doi.org/10.1146/annurev.arplant.49.1.611
PMid:15012248
Shanklin J., and Somerville C., 1991, Stearoyl-acyl-carrier-protein desaturase from higher plants is structurally unrelated to the animal and fungal homologs, Proceeding of the National Academy of Sciences of the United States of America, 88: 2510-2514
https://doi.org/10.1073/pnas.88.6.2510
PMid:2006187 PMCid:PMC51262
Shockey J., Regmi A., Cotton K., Adhikari N., and Browse J., 2016, Bates P D. Identification of Arabidopsis GPAT9 (At5g60620) as an essential gene involved in triacylglycerol biosynthesis, Plant Physiology, 170(1): 163-179
https://doi.org/10.1104/pp.15.01563
PMid:26586834 PMCid:PMC4704598
Taylor M.A., Smith S.B., Davies H.V., Buth L.R., 1992, The primary structures of the precursor of a cDNA clone of the stearoyl-acyl carrier protein desaturase gene from potato (Solanum tuberosum L.), Plant Physiology, 100: 533-534
https://doi.org/10.1104/pp.100.1.533
PMid:16652995 PMCid:PMC1075583
Wang M., Li X.Z., Ning D.L., Zhang Y., and Li Y.J., 2009, The research summary and developmental trend of Carya illinoensis, Forest Inventory and Planning, 34(6): 93-95
Wang M., Ning D.L., Li X.Z., Zhang Y., and Li Y.J., 2010, The survey research and development trends of Carya illinoensis, Forest By-Product and Speciality in China, 105(2): 84-86
Xiong X.H., Guan C.Y., Li X., Wang X.J., Zhou X.Y., and Li J.Y., 2002, Cloning of a sequence encoding fad2 from Bassica napus and constructing of antisence fad2 expression vector, Chinese Journal of Oil Crop Science, 24(2): 1-4
Zaborowska Z., Starzycki M., Femiak I., Swiderski M., and Legocki A.B., 2002, Yellow lupine gene encoding stearoyl-ACP desaturase-organization, expression and potential application, Acta Biochimica Polonica, 49: 29-42
https://doi.org/10.18388/abp.2002_3818
PMid:12136953
Zhang D., Tan X., and Hu F., 2008, The cDNA cloning and characteristic of stearoyl-ACP desaturase gene of Camellia Oleifera, Acta Horticulturae, 769(769): 55-61
https://doi.org/10.17660/ActaHortic.2008.769.5
Zhang D.Q., Tan X.F., Cheng H.P., Zeng Y.L., Jiang Y., Li W., and Hu F.M., 2008, Full-length cDNA cloning and bioinformatic analysis of Camellia oleifera SAD, Scientia Silvae Science, 44(2): 155-159
Zhang Y., 2011, Clone and functional verification of Δ9 - and Δ15 -fatty acid desaturase gene from Lipomyces kononenkoae, Wuhan: Huazhong Agricultural University
Zhao N., Zhang Y., Li Q., Li R., Xia X., Qin X., and Guo H., 2015, Identification and expression of a stearoyl-ACP desaturase gene responsible for oleic acid accumulation in Xanthoceras sorbifolia seeds, Plant Physiology Biochemistry, 87(5): 9-16
https://doi.org/10.1016/j.plaphy.2014.12.009
PMid:25528221
Zhao N., Zhang Y., Li Q.Q., Li R.F., and Guo H.H., 2015, Sequence and functional analysis of FAD2 gene from Xanthoceras sorbifolia seeds, Journal of Beijing Forestry University, 37(2): 87-93
. PDF(2478KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Zhanhui Jia
. Xiaodong Jia
. Mengyang Xu
. Zhenghai Mo
. Xufeng Yang
. Min Zhai
. Jiping Xuan
. Jiyu Zhang
. Gang Wang
. Tao Wang
. Zhongren Guo
Related articles
. Carya illinoensis (Wangech.) K. Koch
. Oil metabolism
. Gene cloning
. Bioinformatics analysis
Tools
. Email to a friend
. Post a comment
.png)


.png)
.png)
.png)
.png)
.png)