2Department of Pharmaceutical Sciences, Assam University Silchar-788011, India
 Author
Author  Correspondence author
Correspondence author
Bioscience Methods, 2015, Vol. 6, No. 2 doi: 10.5376/bm.2015.06.0002
Received: 05 May, 2015 Accepted: 10 Aug., 2015 Published: 12 Aug., 2015
Rohit Sharma and Sarabjot Kaur., 2015, Comparative Analysis of Phenolic Content and Anti-oxidant Activity of Dietary Vegetables, Bioscience Methods, Vol.6, No.2 1-7 (doi: 10.5376/bm.2015.06.0002)
The anti-oxidant activity and total phenolic contents of alcoholic extracts from 14 vegetables were evaluated by using a model system consisting of β-carotene and linoleic acid and Folin-Ciocalteu method. The total phenolic of the extracts was determined spectrophotometrically according to the Folin-Ciocalteu procedure and ranged from 63 to 33 mg per 100 gm on a fresh weight basic. Chenopodium album, Beta vulgaris, Brassica juncea, pea pods, and Brassica oleracea have high anti-oxidant activity. The anti-oxidant activity expressed as per percent inhibition of oxidation ranged from a high of 70% in Chenopodium album extracts to a low of 25% in Raphanus sativus. Other vegetables found to have high anti-oxidant activity (>60%) were Beta vulgaris, Brassica juncea, pea pods, Brassica oleracea. Anti-oxidant activity correlated linearly significantly and positively with total phenolics. The results indicate that vegetables containing high phenolics may provide a source of dietary anti-oxidants.
1 Introduction
Plants consumed by humans may contain thousands of different phenolic compounds. The effects of dietary phenolics are of great current interest, due to their anti -oxidative and possible anti -carcinogenic activity. Phenolic compounds also function as free-radical scavengers, reducing agents, and quenchers of singlet-oxygen formation. Ancient plants are still in use today. They are taken as remedies for cough (ginger), intestinal bleeding (pomegranate), diarrhea (banana) and other medicinal conditions. These plants attracted the interest of many scholars, such as botanists, biochemists and pharmacognosist (natural drug specialists). They are all interested to find out the health benefits and the health promoting effects of these plants and the nature of the active principals they possess. (Wong et al., 2006) One of the documented health promoting activities of many fruits and vegetables is their ability to scavenge naturally produced free radicals and hence acting as antioxidants. Free radicals are normally generated in substantial amounts as a by-product of various internal metabolic processes in aerobic organisms such as phagocytosis, neutrophils defense, auto oxidation of catecholamine and carboxylation or hydroxylation reactions. All of these processes happen in various ways at different times and site (Jose, 2013).
Antioxidants are compounds that can delay or inhibit the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidizing chain reactions. The antioxidant activity of phenolic compounds is mainly due to their redox properties, which can play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides. In general, there are two basic categories of antioxidants, natural and synthetic. Recently, interest has increased considerably in finding naturally occurring antioxidants for use in foods or medicinal materials to replace synthetic antioxidants, which are being restricted due to their carcinogenicity (Kaur et al., 2002).
Over the past 10 years, researchers and food manufacturers have become increasingly interested in polyphenols. The chief reason for this interest is the recognition of the antioxidant properties of polyphenols, their great abundance in our diet, and their probable role in the prevention of various diseases associated with oxidative stress, such as cancer and cardiovascular and neurodegenerative diseases. Furthermore, polyphenols, which constitute the active substances found in many medicinal plants, modulate the activity of a wide range of enzymes and cell receptors. There is now overwhelming evidence to indicate that free radicals and oxygen species causing oxidative damage to lipid, protein and nucleic acid. Therefore, they have been implicated in the pathogenesis of many human sufferings like cardiovascular and pulmonary diseases, some types of cancer, cataracts, immune / autoimmune diseases, inflammation, arthritis, atherosclerosis and brain dysfunction (Parkinson’s, Alzheimer’s, Huntington’s diseases) (Thamizhvanan et al., 2012).
Allium sativum extracts are also used as potential cardiovascular and anticancer agents (Ghasemzadeh et al., 2010). Mushrooms, white Brassica oleracea, cauliflower and Allium sativum have also been shown to have strong protective activity against a number of diseases (kamonrat et al., 2010). Extracts of many vegetables have anti-mutagenic effects. Beta vulgaris is regarded, as the ‘Brain Food’ needed to avoid memory loss and Alzheimer disease. Broccoli is a potential source of glucosinolates having anti-cancerous activity (Saffa et al., 2010).
However, numerous studies have conclusively shown that the majority of the antioxidant activity may be from compounds such as flavonoids, iso-flavone, flavones, anthocyanin, catechin and iso-catechin rather than from Vitamin C, E and b-carotene (Wang et al., 1996). Epidemiological studies have shown that consumption of food and beverages rich in phenolic content can reduce the risk of heart disease by slowing the progression of atherosclerosis by acting as anti-oxidants towards low-density lipoprotein (LDL) (Yafang et al., 2010). Therefore, mostly, the current focus is on the anti-oxidant action of phenolics. The anti-oxidant activity of phenolics is mainly because of their redox properties which allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers and metal chelators (Ljiljana et al., 2009). Elimination of synthetic anti-oxidants in food applications has given more impetus to exploring natural sources of anti-oxidants. In this context a large number of plant sources including many vegetables and fruits have been explored for their anti-oxidant potential (Oviasogic et al., 2009). Mushroom, white Brassica oleracea and cauliflower (Mohammed and Aly, 2008), Allium sativum, broccoli, kidney and pinto beans (Patricia et al., 2008), beans, beet and corn have been reported to have high anti-oxidant activity. Other vegetables such as Brassica juncea leaves, Beta vulgaris, Chenopodium album, alfalfa sprouts, broccoli, beets, red bell-pepper, Allium cepa, corn, and Cucumis sativus are also rich source of anti-oxidant (Kaur et al., 2002).
Green leafy vegetables (GLV) are rich sources of many nutrients and form a major category of vegetable groups that have been designated as ‘nature’s anti-aging wonders’. Therefore, the objective of the present study was to determine the antioxidant activity of these GLV using in vitro models and their correlation with their total polyphenol, β-carotene contents. (Kaur et al., 2002) Human have evolved highly complex anti-oxidant systems (enzyme and non-enzyme), which work synergistically, and in combination with each other to protect the cells and organ systems of the body against free radical damage. The anti-oxidants can be endogenous or obtained exogenously e.g. as a part of a diet or as dietary supplements. Some dietary compounds that do not neutralize free radicals, but enhance endogenous activity may also be classified as anti-oxidants (Lien et al., 2008).
An ideal anti-oxidant should be readily absorbed and quench free radicals, and chelate redox metals at physiologically relevant levels. It should also work in both aqueous and/or membrane domains and effect gene expression in a positive way. Endogenous anti-oxidants play a crucial role in maintaining optimal cellular functions and thus systemic health and well-being. However, under conditions, which promote oxidative stress, endogenous anti-oxidants may not be sufficient and dietary antioxidants may be required to maintain optimal cellular functions. (Wong et al., 2005) The most efficient enzymatic antioxidants involve glutathione peroxidase, catalase and superoxide dismutase. Non-enzymatic antioxidants include Vitamin E and C, thiol antioxidants (glutathione, thioredoxin and lipoic acid), melatonin, carotenoids, natural flavonoids, and other compounds. Some antioxidants can interact with other antioxidants regenerating their original properties; this mechanism is often referred to as the “antioxidant network”. There is growing evidence to support a link between increased levels of ROS and disturbed activities of enzymatic and non-enzymatic antioxidants in diseases associated with aging (Rahman et al., 2007)
In the present study, the anti-oxidant activity and phenolic content of different vegetables that are grown in India and consumed in the Indian diet will be evaluated. Therefore main objectives of present study are to determine total phenolic content and anti-oxidant activity of fourteen vegetables and correlate the phenolic content and antioxidant activity of these vegetables.
2 Results and Discussion
2.1 Total phenolics
The results of phenol analysis of 14 commonly consumed vegetables in India are given in Tables 1, 2 and 3, respectively. Total phenolic content of the vegetables varied from 54.4 mg catechol/100 g fresh weight in Chenopodium album to 34 mg catechol/100 g fresh weight in Raphanus sativus. Considering a large variation in the total phenolics, the vegetables were divided into three groups namely high (>40 mg catechol/100 g), medium (30-40 mg catechol/100 g) and low (<30 mg catechol/100 g).
|
Table 2 Vegetables of medium antioxidant activity and total phenolic content
|
|
Table 3 Vegetables of low antioxidant activity and total phenolic content
|
Chenopodium album, Beta vulgaris, Brassica juncea, pea pods, Brassica oleracea had the highest fresh weight concentration of total phenols followed by carrot leaves, Brassica rapas, French beans, Allium cepa, and Allium sativum. As Beta vulgaris is a rich source of the carotenoids lutein and zeaxanthin; also a good beta carotene and vitamin C source. In one study, eating a lot of Beta vulgaris or collard greens was associated with reduced risk of the leading cause of blindness over 65 (Bayani et al., 2009).
Cruciferous vegetable Brassica juncea green is rich in dithiolthiones, isothiocyanates, the carotenoids lutein and zeaxanthin, some beta carotene, flavonoids and organosulfides. They also contain glucarates, coumarins and other phenolic acids, terpenes and vitamin C (Emerit et al., 2004).
Brassica oleracea is having Indoles, dithiolthiones, isothiocyanates, flavonoids, organosulfides, glucarates, coumarins and other phenolic acids, terpenes, selenium and vitamin C and Pisum sativumare the Modest source of carotenoids; dietary fiber. Due to the present of these anti-oxidant compounds, they are having high anti-oxidant activity and high phenolic contents (Bayani et al., 2009).
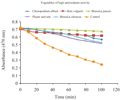
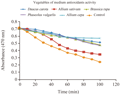
A large variation in the anti-oxidant activities, ranging from as high as 70.8% in Chenopodium album to low as 25.7% in Raphanus sativus, was observed. List of vegetables which are having medium anti-oxidant activity and phenolic contents are categorized in Table 2. These vegetables are Brassica rapa; Daucus carotaAllium cepa, Allium sativum, French beans. Anti-oxidant activity measured by bleaching of linoleic acid-carotene emulsion for high anti-oxidant activity group in Figure 2, for medium anti-oxidant activity group in Figure 3 and for low anti-oxidant activity group in Figure 4).
|
Figure 1 Graph representing the standard curve by using catechol as a standard for calculating the total phenolic content in vegetables samples
|
|
Figure 2 Anti-oxidant activity measured by bleaching of linoleic acid-carotene emulsion (high anti-oxidant activity group)
|
|
Figure 3 Anti-oxidant activity measured by bleaching of linoleic acid-carotene emulsion (medium anti-oxidant activity group)
|
|
Figure 4 Anti-oxidant activity measured by bleaching of linoleic acid-carotene emulsion (low anti-oxidant activity group)
|
2.2 Relationship between phenolic content and anti-oxidant activity
The relationship between total phenolic content and anti-oxidant activity of vegetables is shown in Figure 5. Recently (Patricia et al., 2008) and (Ravi et al., 2006) have demonstrated a linear relationship between anti-oxidant capacity and total phenolics in Rubus sp. However, (Saffa et al., 2010) could not find any correlation between total phenolic content and anti-oxidant activity of the plant extracts. According to them different phenolic compounds have different responses in the Folin-Ciocalteu method. Similarly the molecular anti-oxidant response of phenolics in methyl linoleate varies remarkably depending on their chemical structure (Anna et al., 2008). Thus the anti-oxidant activity of an extract could not be explained just on the basis of their phenolic content but also required their proper characterization. Another Japanese study used the Folin assay for fresh vegetable extracts and measured their activity using β-carotene bleaching coupled with the oxidation of linoleic acid (Tsushida et al., 1994). They found a positive correlation of anti-oxidant activity with phenol content. This correlation suggests that although the vegetables may contain other anti-oxidants such as proteins, ascorbate and the carotenoids, these do not contribute significantly to the anti-oxidant activity (Patel et al., 2010), corroborated this assumption.
According to the results, a positive correlation of anti-oxidant activity with phenol content was found. This correlation suggests that most of the vegetables which are having high antioxidant activity may also show high phenolic contents. Although the vegetables may contain other anti-oxidants such as proteins, ascorbate and the carotenoids, these do not contribute significantly to the anti-oxidant activity. Results are represented graphically in Figure 5, with anti-oxidant activity correlated significantly and positively with total phenolics (r2 = 0.6947, P < 0.05). The results indicate that vegetables containing high phenolics may provide a source of dietary anti-oxidants.
|
Figure 5 The relationship between total phenolic content and anti-oxidant activity of vegetables
|
3 Conclusion
The study reported the antioxidant activity and total phenolic contents of fourteen vegetables. The study clearly indicates that it is important to measure the anti-oxidant activity using various radicals and oxidation systems and to take both phenolic content and anti-oxidant activity into account while evaluating the anti-oxidant potential of plant extracts. However, the model system consisting of β-carotene and linoleic acid can be used to screen large number of sources for their anti-oxidant capacity. Furthermore, in order to realize the health benefits from potential plant sources, additional information on their dietary intake and enhancing bioavailability after various processing operations is required. Currently work is underway in our laboratory to confirm the anti-oxidant activity of various fruit and other sources by using other oxidation systems and lipid models. In addition more work on optimizing processing and storage conditions for their maximum stabilization is also under progress. Phenolic compounds could be a major determinant of antioxidant potentials of food plants and could therefore be a natural source of antioxidants and because Phenolic compounds have been associated with the health benefits derived from consuming high levels of vegetables.
Research on polyphenol bioavailability must finally allow us to correlate polyphenol intakes with one or several accurate measures of bioavailability (such as concentrations of key bioactive metabolites in plasma and tissues) and with potential health effects in epidemiologic studies. Knowledge of these correlations must be attained despite the difficulties linked to the high diversity of polyphenols, their different bio availabilities, and the high inter individual variability observed in some metabolic processes, especially those in which the microflora is involved.
4 Materials and Methods
4.1 Sample preparation
Fourteen vegetables were purchased fresh from different market. These were cleaned, washed and chopped into small pieces. Allium cepas, Allium sativum, Brassica rapa, Raphanus sativus, and other vegetables having dry skins were processed after removal of their skins. Only edible portions of vegetables were weighed and homogenized using a pestle motor.
Ethanol extracts of leaves of leafy vegetables as well as root extracts was prepared. Phenolic content in these vegetables (Beta vulgaris, Brassica juncea, Chenopodium, Brassica rapa, Raphanus sativus, Allium cepa, Allium sativum, French beans, Pea pods, green chilies, Brassica oleracea, Brassica oleracea and Cucumis sativus) was measured using Folin-Ciocalteau procedure. Anti-oxidant activity in these vegetables was measured using β-carotene bleaching method. Comparative analysis of anti-oxidant activity and phenolic content in these vegetables shows positive relationship.
4.2 Determination of total phenolic content
Folin-Ciocalteu procedure
Total phenols were determined using the Folin-Ciocalteu reagent (Kaur et al., 2002). Samples (2 g) were homogenized in 80% aqueous ethanol at room temperature and centrifuged in cold at 10,000 rpm for 15 min and the supernatant was saved. The residue was re-extracted twice with 80% ethanol and supernatants were pooled, put into evaporating dishes and evaporated to dryness at room temperature. Residue was dissolved in 5 mL of distilled water. One-hundred microliters of this extract was diluted to 3 mL with water and 0.5 ml of Folin-Ciocalteu reagent was added. After 3 min, 2 mL of 20% of sodium carbonate was added and the contents were mixed thoroughly. The color was developed and absorbance measured at 650 nm in spectrophotometer after 60 min using catechol as a standard. The results were expressed as mg catechol/100 g of fresh weight material.
Phenolic content can be calculated as Where, O.D sample is Optical density of sample, DF is Dilution Factor, and A650 is Absorbance of standard at 650 nm,
.jpg)
4.3 Determination of anti-oxidant activity
Beta carotene bleaching method
For the determination of anti-oxidant activity, alcoholic (80%) vegetable extracts were prepared. Vegetable ( 2 g ) were homogenized in the respective media and centrifuged at 10,000 rpm for 15 min. Supernatants were stored in capped tubes until further use. Anti-oxidant activity was determined according to the b-carotene bleaching method as described by (Anna et al., 2008) with modifications. β- Carotene (2 mg) was dissolved in 20 ml of chloroform. A 4 ml aliquot of the solution was added to a conical flask with40 mg linoleic acid and 400 mg Tween-40. Chloroform was removed with a rotary evaporator at 50℃. Oxygenated distilled water (100 ml) was added to the b-carotene emulsion mixed well and aliquots (3 ml) of the oxygenated b-carotene emulsion and 0.2 ml of water/alcoholic extracts were placed in capped culture tubes and mixed well. The tubes were immediately placed in a water bath and incubated at 50℃. Oxidation of the β-carotene emulsion was monitored with spectrophotometer taking absorbance at 10-min interval at 470 nm for 100 min. A control consisted of 0.2 ml distilled water instead of vegetable extract.
Anti-oxidant activity was expressed as per cent inhibition relative to control using the equation
![]()
Where: Abssample 0min is Absorbance of sample at 0 minute, Abs sample100min is Absorbance of sample at 100 minute, Abs control 0min is Absorbance of control at 0 minute and Abs control 100min is Absorbance of control at 100 minute,
4.4 Procedure for standard curve
Prepared a stock solution of 10mg of catechol in 100 ml of distilled water, 0.2ml, 0.4ml, 0.6ml, 0.8ml and 1ml of this solution were diluted to 3 ml with water and 0.5 ml of Folin-Ciocalteu reagent was added. After 3 min, 2 ml of 20% of sodium carbonate was added and the contents were mixed thoroughly. The color was developed and absorbance measured at 650 nm spectrophotometer after 60 min. Figure 1 shows the standard curve by using catechol as a standard for calculating the total phenolic content in vegetables samples.
Acknowledgement
Authors are extremely grateful to the Chancellor, Dean and entire Biotechnology department, Lovely Professional University for the support.
References
Wong S. P., leong L. P. and William J. H., 2006, Antioxidant activities of aqueous extracts of selected plants food science and technology program me, Food Chemistry, 99: 775-783
http://dx.doi.org/10.1016/j.foodchem.2005.07.058
Jose A. M. G., 2013, Oxidative Stress and Chronic Degenerative Diseases - A Role for Antioxidants, Published by In Tech, DOI: 10.5772/45722
http://dx.doi.org/10.5772/45722
Ghasemzadeh A., Hawa, J. and Rahmat A., 2010, Antioxidant activities, total phenolics and flavonoids content in two varieties of malaysia young ginger (zingiber officinale roscoe), Journal of Medicinal Plants Research, 4: 881-890
http://dx.doi.org/10.3390/molecules15064324
Kaur C. and Kapoor C. H., 2002, Anti-oxidant activity and total phenolic content of some asian vegetables, International Journal of Food Science & Technology, 37(2):153-161
http://dx.doi.org/10.1046/j.1365-2621.2002.00552.x
Thamizhvanan K., Kumuda P. and Sateesh B., 2012, Evaluation of antioxidant activity of whole plant of ipomoea eriocarpa extract, International Journal of Innovative Drug Discovery, 2: 1-3
Kamonrat R., Lalida S. and Griangsak C., 2010, Phenolic content and antioxidant properties of green chilli paste and its ingredients, International Journal of Science and Technology, 4:193-200
Safaa Y. Q., Ahmed N. and Mona A. B. L., 2010, Screening of antioxidant activity and phenolic content of selected food items cited in the Holy Quran, Journal of Biological Science, 2:40-51
Patel V.R. and Patel P.R., 2010, Antioxidant Activity of Some Selected Medicinal Plants in Western Region of India, Advances in Biological Research, 4: 23-26
Yafang S., Liang J., Gan Z., Yan L., Yun S. and Jinsong B., 2010, Association mapping of grain color, phenolic content, flavonoid content and antioxidant capacity in dehulled rice, 122:1005-10016
Ljiljana S. , Mihajlo S., Vesna N., Ljubisa N., Dusica R J., Canadanovic B. and Vesna T., 2009, Antioxidant Activity and Total Phenolic and Flavonoid Contents of Hieracium pilosella L. Extracts, 9: 5702-5714
Oviasogie P. O., Okoro D. and Ndiokwere C., 2009, Determination of total phenolic amount of some edible fruits and vegetables, African Journal of Biotechnology, 8: 2819-2820
Mohamed A. A. and Aly A. A., 2008, Alterations of some secondary metabolites and enzymes activity by using exogenous antioxidant compound in Allium cepa plants grown under seawater salt stress,” American-Eurasian Journal of Scientific Research, 3:139-146
Anna L. H., Lavanya R., Ndambe M. N., John B. B. and Miller J. C., 2008, Interspecific Variability for Antioxidant Activity and Phenolic Content among Solanum Species, American journal of potato research, 85:332-341
http://dx.doi.org/10.1007/s12230-008-9035-1
Bukhari S.B, Bhanger M. I and Memon S., 2008, Anti-oxidative activity of extracts from fenugreek seeds (trigonella foenum-graecum), Pak. J. Anal. Environ. Chem, 9: 78-83
Patricia M., Suhaila M., Noordin M., Mustapha, Kharidah M., and Cheng H. M., 2008, Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo, 20: 367-373
Lien A., Hua H., and Chuong P.H., 2008, Free Radicals, Antioxidants in Disease and Health, Int J Biomed Sci. 4: 89-96
Rahman K., 2007, Studies on free radicals, antioxidants, and co-factors, Clin Interv Aging, 2(2): 219-236
Bayani U., Singh A. V., Paolo Z. and Mahajan R.T., 2009, Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options, Curr Neuropharmacol, 7(1): 65-74
http://dx.doi.org/10.2174/157015909787602823
Emerit J., Edeas M. and Bricaire F., 2004, Neurodegenerative diseases and
oxidative stress, Biomed Pharmacother, 58:39-46
http://dx.doi.org/10.1016/j.biopha.2003.11.004
Ravi P. N. M., Singh R K., Jaiswal H. K., Kumar V. and Maurya S., 2006, Rhizobium-Mediated Induction of Phenolics and Plant Growth Promotion in Rice ( Oryza sativa L.), Current microbiology, 52: 383-389
http://dx.doi.org/10.1007/s00284-005-0296-3
Ansari N. M., Houlihan, H. and Pieroni A., 2005, Antioxidant activity of five vegetables traditionally consumed by South-Asian migrants in Bradford, Yorkshire, UK, 19: 907-911
. PDF(586KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Rohit Sharma
. Sarabjot Kaur
Related articles
. β-carotene
. linoleic acid
. Folin-Ciocalteu reagent
. green leafy vegetables
Tools
. Email to a friend
. Post a comment